
UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 10-K ☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 2022 OR ☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 Commission File No. 001-37854 Ekso Bionics Holdings, Inc. (Exact name of registrant as specified in its charter) Nevada 99-0367049 (State or Other Jurisdiction of Incorporation or Organization) (I.R.S. Employer Identification No.) 101 Glacier Point, Suite A San Rafael, California 94901 (Address of Principal Executive Offices) (Zip Code) Registrant's telephone number, including area code: (510) 984-1761 Securities registered pursuant to section 12(b) of the Act: Title of each class Trading Symbol Name of each exchange on which registered Common Stock, $0.001 par value EKSO Nasdaq Stock Market LLC (Nasdaq Capital Market) Securities registered pursuant to section 12(g) of the Act: None Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No ý Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No ý Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No ¨ Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ý No ¨ Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and "emerging growth company" in Rule 12b-2 of the Exchange Act. Large accelerated filer ¨ Accelerated filer ¨ Non-accelerated filer ý Smaller reporting company ý Emerging growth company ☐ If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨ Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ¨ If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐ Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐ Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒ The aggregate market value of the registrant's common stock held by non-affiliates of the registrant was $18,860,934 based on the last sale price for such stock on June 30, 2022, the last business day of the registrant's most recently completed second fiscal quarter. As of March 23, 2023 the registrant had 13,341,505 outstanding shares of common stock. DOCUMENTS INCORPORATED BY REFERENCE: Portions of the registrant’s Proxy Statement for the 2023 Annual Meeting of Stockholders are incorporated herein by reference in Part III of this Annual Report on Form 10-K to the extent stated herein. Such proxy statement will be filed with the Securities and Exchange Commission within 120 days of the registrant’s fiscal year ended December 31, 2022. Ekso Bionics Holdings, Inc. ANNUAL REPORT ON FORM 10-K For the Year Ended December 31, 2022 Table of Contents Part I Item 1 Business 4 Item 1A Risk Factors 19 Item 1B Unresolved Staff Comments 37 Item 2 Properties 37 Item 3 Legal Proceedings 37 Item 4 Mine Safety Disclosures 37 Part II Item 5 Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities 38 Item 6 Reserved 38 Item 7 Management’s Discussion and Analysis of Financial Condition and Results of Operations 39 Item 7A Quantitative and Qualitative Disclosures About Market Risk 47 Item 8 Financial Statements and Supplementary Data 48 Item 9 Changes in and Disagreements With Accountants on Accounting and Financial Disclosure 85 Item 9A Controls and Procedures 85 Item 9B Other Information 85 Part III Item 10 Directors, Executive Officers and Corporate Governance 86 Item 11 Executive Compensation 86 Item 12 Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters 86 Item 13 Certain Relationships and Related Transactions and Director Independence 86 Item 14 Principal Accountant Fees and Services 86 Part IV Item 15 Exhibits, Financial Statements and Financial Statement Schedules 87 Item 16 10-K Summary 91 Signatures 92 Table of Contents 2 CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS This Annual Report on Form 10-K, or this Annual Report, contains forward-looking statements, including, without limitation, in the sections captioned “Business,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and elsewhere. Any and all statements contained in this Annual Report that are not statements of historical fact may be deemed forward-looking statements. Terms such as “may,” “might,” “would,” “should,” “could,” “project,” “estimate,” “pro-forma,” “predict,” “potential,” “strategy,” “anticipate,” “attempt,” “develop,” “plan,” “help,” “believe,” “continue,” “intend,” “expect,” “future,” and terms of similar import (including the negative of any of the foregoing) may be intended to identify forward-looking statements. However, not all forward-looking statements may contain one or more of these identifying terms. Forward-looking statements in this Annual Report may include, without limitation, statements regarding (i) the plans and objectives of management for future operations, including plans or objectives relating to the design, development and commercialization of exoskeleton products for humans, (ii) the manufacturing of our products and strengthening our supply chain, and potential opportunities for strategic partnerships, (iii) beliefs regarding regulatory path for our products, including potential approvals required and timing of approvals, (iv) statements regarding the financial and operational impacts on our business following the completion of our acquisition from Parker Hannifin Corporation of certain assets related to Parker Hannifin Corporation's human motion control business, and software applications, support services and cloud environments related to such business in December 2022 (the "HMC Acquisition"), (v) our future financial performance, including any statement contained in a discussion and analysis of our financial condition by management or in the results of operations included pursuant to the rules and regulations of the Securities and Exchange Commission ("SEC"), (vi) our beliefs regarding the potential for commercial opportunities, including for exoskeleton technology and, our exoskeleton products, and for strategic partnerships, (vii) our beliefs regarding potential clinical and other health benefits of our medical devices, (viii) the impact and effects of the COVID-19 pandemic and other risk factors on our business, results of operations or prospects, and (ix) the assumptions underlying or relating to any statement described in points (i) through (ix) above. The forward-looking statements are not meant to predict or guarantee actual results, performance, events or circumstances and may not be realized because they are based upon our current projections, plans, objectives, beliefs, expectations, estimates and assumptions and are subject to a number of risks and uncertainties and other influences, many of which we have no control over. Actual results and the timing of certain events and circumstances may differ materially from those described by the forward-looking statements as a result of these risks and uncertainties. Factors that may influence or contribute to the inaccuracy of the forward-looking statements or cause actual results to differ materially from expected or desired results may include, without limitation, the ongoing COVID-19 pandemic and its impact on the Company’s financial condition and business, the highly competitive markets in which the Company’s products are sold, the Company's significant losses to date and anticipated future losses, the new and unproven nature of the market for the Company’s products, the long and variable sales cycles for the Company’s products, the factors outside the Company’s control that affect the production and sales of its products, which include but are not limited to disruptions in the global supply chain, the costs related to and impacts of potential failure of the Company to obtain or maintain protection for the Company's intellectual property rights, the inability to successfully consummate and integrate acquisitions, including the HMC Acquisition, the failure of the Company to obtain or maintain regulatory approval to market the Company's medical devices, risks related to product liability, recall and warranty claims, the volatility of the market price of and limited trading in our common stock. A description of some of the risks and uncertainties that could cause our actual results to differ materially from those described by the forward-looking statements in this Annual Report appears in the section captioned “Risk Factors” and elsewhere in this Annual Report. Readers are cautioned not to place undue reliance on forward-looking statements because of the risks and uncertainties related to them and to the risk factors. We disclaim any obligation to update the forward-looking statements contained in this Annual Report to reflect any new information or future events or circumstances or otherwise. Readers should read this Annual Report in conjunction with the discussion under the caption “Risk Factors,” our financial statements and the related notes thereto in this Annual Report, and other documents which we may file from time to time with the SEC. Table of Contents 3

PART I Item 1. BUSINESS Overview We design, develop, and market exoskeleton products that augment human strength, endurance and mobility. Our exoskeleton technology serves multiple markets and can be utilized both by able-bodied persons and persons with physical disabilities. We have marketed devices that (i) enable individuals with neurological conditions affecting gait, including acquired brain injury (ABI) and spinal cord injury (SCI), to rehabilitate, and in some cases, to walk again, (ii) provide ambulation assistance in home and community settings for individuals with certain SCI levels, (iii) assist individuals with a broad range of upper extremity impairments, and (iv) allow industrial workers to perform difficult repetitive work for extended periods. We believe that the commercial opportunity for exoskeleton technology adoption is accelerating as a result of recent advancements in material technologies, electronic and electrical engineering, control technologies, and sensor and software development. Taken individually, many of these advancements have become ubiquitous in peoples’ everyday lives. We believe that we have learned how to integrate these existing technologies and wrap the result around a human being efficiently, elegantly, and safely, supported by an industry leading intellectual property portfolio. We further believe that we can do so across a broad spectrum of applications, from persons with lower limb paralysis to able-bodied users. On December 5, 2022, we acquired the Human Motion and Control (“HMC”) Business Unit from Parker Hannifin Corporation (“Parker”), an Ohio corporation (the "HMC Acquisition"). The assets acquired from the business unit include intellectual property rights for devices which are U.S. Food and Drug Administration (FDA) cleared lower-limb powered exoskeletons that enable task-specific, overground gait training to patients with weakness or paralysis in their lower extremities. The HMC acquisition has the potential to fuel growth by increasing top line revenue and expanding our reach throughout the continuum of care. We continue to explore business development initiatives to fuel growth and long-term value and are committed to helping people improve mobility and live healthier lives through combining the use of technology with advanced rehabilitative programs. For medical applications we have three main products. • EksoNR is a robotic exoskeleton specifically designed to be used in a rehabilitation setting to progress neurorehab patients so they can walk out of the device and back into their communities. As an exoskeleton FDA-cleared for acquired brain injury, stroke, multiple sclerosis (MS) and spinal cord injury, EksoNR offers what we believe is the industry’s most natural gait, re-teaching the brain and muscles how to properly walk again. • Ekso Indego Therapy is a modular, adjustable, lightweight, lower-limb powered exoskeleton that can be custom-sized and fitted to patients for use in rehabilitation and wellness applications. Ekso Indego Therapy is cleared by the FDA for use with stroke and SCI patients. • Ekso Indego Personal is a lightweight powered lower limb orthosis that enables people with mobility impairments the opportunity to walk independently. Ekso Indego Personal is cleared by the FDA for use with SCI patients with injury levels from T3 to L5 in community or home settings. For able-bodied industrial workers, we have offered three products. • Ekso EVO, a wearable exoskeleton for overhead work. EVO is an upper body exoskeleton that elevates and supports a worker's arms to assist them with tasks from chest height to overhead. EVO is intended to reduce worker fatigue and reduce on-site injuries while boosting productivity. Based on extensive customer feedback, we have engineered EVO to be light weight, have a low profile, allow for minimal contact with the body, and offer a wide range of uninhibited arm motion. EVO is currently targeted at vertical markets including aerospace, automotive, manufacturing, and specific construction trades. • EksoVest is the predecessor product to EVO and has similar properties and applications. EksoVest was discontinued in 2022. • EksoZeroG is a mobile tool support arm that can be mounted on a fixed structure to reduce the load transferred from the tool to the user. EksoZeroG is used primarily in construction and demolition applications. EksoZeroG was discontinued in 2022. Table of Contents 4 EksoHealth - Rehabilitation Today, we focus our healthcare business on advanced technology in the rehabilitation market. We are leveraging our patented exoskeleton technology to develop and market products intended to rehabilitate patients earlier and with better outcomes than the current standard of care. As of December 31, 2022, we had shipped approximately 620 EksoNR and EksoGT units combined to over 400 rehabilitation facilities or customers worldwide. The number of units utilized at a facility varies from one to seven, and is driven by the number of beds and rehabilitation sessions a hospital can offer and that hospital’s adoption of robotics within its rehabilitation protocols. As of December 31, 2022, more than 300 Ekso Indego Therapy and Personal devices have been shipped to over 220 clinical centers or personal end users. The number of units at a center may vary for clinical sites based on the size of the site and ability to assist multiple patients simultaneously using Ekso Indego. Some sites that purchased Ekso Indego technology prior to the release of Ekso Indego Therapy received a 3-piece kit of Indego devices, one each of small/short, medium, and large/tall size configurations. EksoNR EksoNR is a wearable bionic exoskeleton that allows hospitals and other rehabilitation providers to deliver over ground gait therapy and ambulation assistance. EksoNR incorporates hardware and software that can provide varying levels of support and assistance to the patient in real-time, correct issues with the patient’s reciprocal gait. Patients receive therapy in the device under the supervision of a physical therapist, and typically use an additional assistive device such as a cane, crutches or a walker. Walking is achieved by a user shifting their weight, requiring the user to achieve balance thereby replicating and reinforcing the movements of a natural gait. Using built-in software, EksoNR's sensors can detect weight shift and initiate steps. Battery-powered motors drive the legs with software determining the appropriate level of assistance necessary for a user to complete the gait sequence. EksoNR is used by customers in both in-patient and out-patient settings. Our customers believe that for patients with some preserved motor ability (for example, after a stroke, an ABI, or an incomplete SCI), EksoNR offers unique benefits. It helps therapists teach proper gait patterns and weight shifts, allowing some patients to potentially mobilize earlier and ultimately to walk again. By allowing individuals to stand and walk in a full weight-bearing setting, early clinical evidence is beginning to show that EksoNR may offer potential healthcare benefits (inclusive of patients with complete SCIs). These benefits include a reduction in secondary complications such as pressure sores, urinary tract infections, bowel problems, pneumonia and other respiratory issues, bone loss/osteoporosis, cardiovascular disease and psychological disorders resulting in reduced post-injury medical costs. EksoNR includes cloud connectivity through EksoPulse, which gathers and transmits statistics and device information during EksoNR walking sessions. This information can be used to track patient progression and to monitor device utilization. Data is sent securely to the cloud where it is available for customers to view, filter, and export through a secure web portal. This feature enables more thorough patient care while reducing manual data entry. It also enables us to provide a higher level of service through early identification and thorough reporting of device errors, saving customers the time and expense of unnecessary on- site visits. Ekso Indego Therapy Ekso Indego Therapy is an adjustable, lower-limb powered exoskeleton that can be custom-sized and tailor fitted to patients allowing for swift donning. A comprehensive software suite further enhances training sessions by providing a variety of options, settings, and analytics on patient and device performance that therapists can use to improve treatment plans in real- time. The Ekso Indego Therapy enables therapists to deliver task specific, over-ground and individualized gait training. Ekso Indego Therapy enables individualized gait therapy for patients with lower extremity weakness or paralysis (such as complete/incomplete spinal cord injury and stroke). Ekso Indego's lightweight, modular, and quick-adjust design allows clinicians to offer intensive gait therapy, custom-tailored to patients across the entire continuum of care from inpatient facilities to in-home sessions and everything in between. Ekso Indego Therapy+ software is designed to provide effective gait therapy for patients with lower extremity weakness, such as partially impaired stroke survivors. Patients are required to initiate leg movement and Ekso Indego provides support when necessary while providing auditory, real-time feedback. Ekso Indego Motion+ software allows clinicians to practice task-specific gait therapy with patients through a predictable, guided gait pattern. Powerful motors in the hip and knee, customizable within the Ekso Indego app, enable patients with little to Table of Contents 5 no gait function to stand and walk with postural controls. The patient leans forward to initiate movement, and Ekso Indego responds accordingly Ekso Indego Personal Ekso Indego Personal is a powered lower limb orthosis, also known as a powered exoskeleton, which enables people with mobility impairments the opportunity to walk independently. Power is provided by sophisticated motors in the knee and hip joints, and combined with advanced sensors and control strategies, the device allows gait impaired individuals to stand and walk again, granting them a new level of independence at home and in the community. Ekso Indego Personal offers a modular quick connect design, which allows its users to put on and take off the device without assistance. At just 29 lb (13 kg), Ekso Indego Personal is a lightweight commercial exoskeleton offering ease of handling, transportation, and storage. Ekso Indego Personal can currently be used with spinal cord injury levels of T3 to L5 in community or home settings, but is not intended for sports or stair climbing. With its slim profile, Ekso Indego Personal offers a modular quick-connect design, which allows users to put on and take off the device without assistance even while seated in most standard-frame wheelchairs. It is compatible with stability aids such as rolling walkers or forearm crutches. With no backpack or exposed wiring, Ekso Indego Personal allows for safe use in most home and community environments and on surfaces like pavement, grass, carpet and tile. Market Overview Rehabilitation clinics with significant stroke, ABI, and SCI populations comprise the primary market for our medical products. Due to their chronic nature, we believe that these conditions have an enormous clinical and economic impact on both people with the conditions and the healthcare system. According to the Centers for Disease Control, there are approximately 800,000 strokes suffered per year in the U.S. and approximately 15 million worldwide, making stroke rehabilitation our largest target market. Likewise, according to the National Spinal Cord Injury Statistical Center, there are approximately 18,000 incidences of SCI per year in the U.S., and according to the World Health Organization, between 250,000 to 500,000 incidences worldwide. We also serve individual users with Ekso Indego Personal, which is intended to provide overground ambulation in community and home settings. The primary use case for Ekso Indego Personal is for users with certain spinal cord injuries. For this patient population, confinement to a wheelchair can cause severe physical and psychological deterioration. As a result, the secondary medical consequences of paralysis can include difficulty with bowel and urinary tract function, osteoporosis, loss of lean mass, gain in fat mass, insulin resistance, diabetes, and heart disease. The cost of treating these conditions is substantial. A particular subset of the SCI population that we address with Ekso Indego Therapy are U.S. veterans treated by the U.S. Department of Veterans Affairs (the "VA"). According to VA data there are approximately 42,000 of such patients are veterans and are eligible for medical care and other benefits from the VA out of which 27,000 are receiving treatment annually. With 25 VA spinal cord injury centers, the VA has the largest single network of spinal cord injury care in the United States. The European Union includes a unique approach to market penetration and subsequent coverage, requiring separate claims for purchasing the device and for requests for reimbursement. We are well represented in clinics run by German and Austrian accident insurers, with four out of nine rehabilitation sites in Germany, and four out of four rehabilitation sites in Austria. We operate out-patient rehabilitation sessions paid for by the accident insurer, where patients train using our devices in rehabilitation setting. We are using these examples to integrate exoskeletal therapy in existing care pathways as well as to pursue personal device sales. While the market opportunity for robotic exoskeleton rehabilitation may be large, we also recognize that the path for medical devices to become the standard of care is long and challenging. We believe that our ability to accelerate adoption will also be based, in part, on our ability to build on our partners’ early efforts to: (i) expand clinical evidence and (ii) drive toward standard of care. We are already seeing customers use our products with patients post stroke, ABI, SCI, or MS to facilitate the recommended amount of rehabilitation per guidelines defined by the American Heart Association. All of our lower extremity products have the versatility to provide an over-ground gait training intervention that is task-specific, high intensity and patient- centered throughout the continuum of care. Clinical Evidence Table of Contents 6 Many of our early clinical customers have participated in research focusing on safety and feasibility of exoskeletons and robotics in rehabilitation market development. These early studies were favorable and have further developed to focus on efficacy, outcomes, dosage, and comparing this technology to other therapeutic interventions. Currently, a search for “robotic exoskeleton” on PubMed, a search engine for biomedical literature and life science journal articles, garners approximately 289 unique publications on the topic. The Ekso exoskeletons (Ekso1.1, EksoGT, EksoNR, and Ekso Indego) have been utilized in many of these protocols. The body of research has been carried out by world-renowned institutions and examines a wide variety of diagnoses including ABI, SCI, stroke, MS, and others. The findings of this research are overall positive and promote use of an Ekso exoskeleton in rehabilitation to provide patient outcomes that are equal to or superior to traditional physical therapy in both the inpatient and outpatient setting. Some of these outcomes include faster gait speed, increased gait endurance, improvements in cardiometabolic responses, enhanced quality of life, more typical gait kinematics, and increased function. More recent research has focused on session duration and demonstrates that patients are able to complete significantly greater numbers of steps using an Ekso exoskeleton than in traditional therapy. This is important because the number of repetitions required to master a skill is high, so being able to utilize this equipment to get more intense practice will lead to improved outcomes. Economic Value Proposition We believe that our EksoNR allows our customers to benefit economically without modifying the reimbursement model or reimbursement codes. First, many of our customers have reported that utilizing the EksoNR promotes continuous patient improvement beginning sooner than with traditional rehabilitation methods, potentially leading to a commensurate increase in insurance reimbursements. Second, many of our customers report that facilities equipped with the EksoNR as part of their rehabilitation programs attract more patients, thereby driving positive economic benefits. Lastly, we believe that improvements in patient outcomes, such as those seen with the use of EksoNR, translate positively to other metrics including discharge to community, staffing efficiency in the rehabilitation unit, and reductions in readmission rates. Ekso Indego Personal addresses the home and community use market for patients with specific spinal cord injuries—further extending the continuum of care beyond the rehabilitation or clinical setting. Today the primary source of revenue for Ekso Indego Personal is from the VA, who purchases devices on behalf of veterans who qualify. Since 2015 the VA has supported coverage for US qualified veterans who have suffered spinal cord injury & further expanded the program in 2018 to support more convenient training options that are closer to patients’ homes. As the industry continues to work to extend Centers for Medicare and Medicaid Services codes for reimbursement through traditional means the market opportunity will expand. Today, in the United States wearable at home assisted exoskeleton medical technology is generally not covered for reimbursement by private insurance providers. However, reimbursement may be approved on a case-by-case basis as in the case of workers compensation or accident settlements. In some instances, devices are paid for by individual users from their own personal funds or through charitable donations or organizations. Additional future commercial opportunities are possible but require more traditional programs for adequate coverage for potential partial or full reimbursement from third party payors, which may include; private health insurance companies, managed care, and government facilities (such as Medicare and Medicaid programs in the United States). Current Sales and Marketing Efforts Our key marketing goal today is the broad-based commercial adoption of our portfolio of robotic wearable exoskeletons, including the EksoNR, Ekso Indego Therapy and the Ekso Indego Personal, in the Hospital and Home setting. We are focusing our go-to-market protocols and collateral on our three target audiences: medical administrators, medical directors/ therapists, and patients. Working closely with thought leaders, we will continue to build upon our early user-group exchanges, develop clinical education programs, and grow our medical advisory council. There continues to be high market interest in expanding neurosciences service lines. In alignment with this interest, our sales priority involves the education of clinical and executive stakeholders on the economic and clinical value of our robotic exoskeleton portfolio, including the EksoNR and the Ekso Indego Therapy devices. In tandem, we continue to leverage our EksoNR and Ekso Indego customer base to educate and mentor strategic target centers that specialize in stroke, ABI and SCI rehabilitation in specific geographies. Geographically, the priorities have been the U.S. in the Americas, Germany in EMEA (the Europe, the Middle East, and Africa region), and Singapore, Hong Kong, and Australia in APAC (the Asia Pacific region). Currently, we utilize a direct sales force for customers located in the U.S., Singapore, Hong Kong, Australia, Germany, Austria and Switzerland. We also have an expanding distributor network in EMEA and APAC. Table of Contents 7

The sales and marketing team is principally based in the U.S., Germany, and Singapore, and is structured as follows: • One commercial leader each for the Americas, EMEA, and APAC; • Americas, EMEA, and APAC sales professionals who pursue new prospects and organize demonstrations; • Clinical professionals and physical therapists who provide peer-to-peer demonstrations and trainings; • Marketing professionals and consultants who build awareness and generate demand; and • Ambassadors, who are stroke and SCI survivors, who provide demonstrations and personal experiences. The sales cycle for the EksoNR and Ekso Indego Therapy devices average approximately eight to 12 months for a first device and six to eight months for subsequent devices. The typical sale of our EksoNR and Ekso Indego Therapy is a complete package, which includes the device and all relevant components, batteries for continuous run-time, training and certification. Customers also typically purchase Ekso Care, which is our one- to four-year after-sales service package. For products sold to hospitals or other rehabilitation clinics, we offer a range of purchase options. In most cases and when capital is available, the product is sold outright to the customer as a capital sale and the full price is invoiced to the customer after title transfers. For customers who prefer to finance the purchase of their device, we have finance partners who facilitate such transactions. Often these arrangements will be marketed as a subscription offer to the end customer. Typically, in a subscription arrangement we will sell the device to the 3rd party financing partner who then contracts with the end customer for payment terms. In some subscription cases we may elect to maintain ownership of the product provided to the customer in lieu of selling it to a 3rd party financing partner. Rehabilitation treatments that can benefit from the use of our EksoNR and Ekso Indego Therapy products take place in a range of different types of facilities. These include inpatient rehab facilities (IRF), long term acute care hospitals (LTACH), skilled nursing facilities (SNF), and outpatient rehab clinics, among others. The primary facility types we currently serve are IRFs. Among these facilities, ownership structures also vary from small independent rehab centers to larger networks of providers. Our current market focus is on the larger network providers, referred to as integrated delivery networks (IDN). Sales to IDNs typically involve multi-unit transactions that can benefit from lower selling costs, better pipeline visibility, and better economies of scale. In 2022, approximately 54% of our new unit shipments for EksoNR were to IDNs, and globally, multi-unit sales comprised approximately 61% of our unit shipments. The sales cycle for the Ekso Indego Personal device averages 8-12 months from the first interaction we have with the potential Personal user. The Ekso Indego Personal device is regulated by the FDA and the patient must have an injury level of T3 to L5 and have a support person when utilizing the device. The majority of Personal users will be Veterans, as we work closely with VA hospitals located throughout the county. The Veteran will need to complete a screening, in-clinic training and a home trial prior to the VA purchasing the device for the Veteran. We sell our medical products through a combination of direct and indirect (i.e., distribution) channels. In the US, our hospital and clinical sales are primarily made through our direct salesforce, with the exception of sales to the VA which are handled through distribution. In EMEA, we sell through a combination of direct and indirect channels, with DACH countries typically handled direct, and other countries and regions served through distributors. In APAC we also use a combination of direct and indirect channels depending on the country. Clinical Services and Customer Success We have developed a leading clinical capability in robotic rehabilitation, and we provide extensive training and support to our customers to ensure they are successful. All sales or subscriptions include customer training. This is comprised of both online and in-person training of our customers’ physical therapists. We have made this a high priority as we recognize getting customers comfortable using our product is a prerequisite to them successfully implementing a robotic rehabilitation program. Product Pipeline As described previously, our current medical products broadly target the rehabilitation and mobility spaces. We believe there are further opportunities in these and adjacent use cases, and we plan to expand our product portfolio accordingly. Our internal medical product development activities are targeted at a combination of next generation versions of our current products as well as new applications in both rehabilitation and mobility. In addition to our internal development activities, we are continuously evaluating complementary external products and services that have the potential to leverage our existing infrastructure and go-to-market capacity to further expand our industry presence. This includes the possibility of pursuing business relationships ranging from acquisitions to licensing activities. Table of Contents 8 EksoWorks - Able-Bodied Industrial Applications We continue to pursue market and product development opportunities for able-bodied industrial applications. Injuries caused by repetitive tasks and overexertion are leading causes of lost work days due to workplace injuries. Ekso Bionics believes that human augmentation and exoskeletons in particular have a key role to play in solving these workplace issues and strives to alleviate the burden on skilled workers, to drastically reduce the number of workplace injuries, and to cut down on worker fatigue. Our primary product for able-bodied industrial applications is EVO, an upper body exoskeleton that elevates and supports a worker's arms to assist them with tasks ranging from chest height to overhead. EVO builds on nearly a decade of design and development history in upper extremity applications and is based on extensive customer feedback. EVO is a passive, spring- loaded assistive upper-body exoskeleton that is designed to reduce fatigue and shoulder and back muscle strain, with the goal of eliminating work-related injuries to the neck, shoulder, and back. EVO offers five to fifteen pounds of lift assistance in each arm to elevate and alleviate the day-to-day strain on workers across all industries. While EVO is a general purpose product, we currently target specific vertical markets including aerospace, automotive, general manufacturing, and some construction trades. EksoVest is a shoulder support product targeted at overhead work. EksoVest was superseded by EVO upon its release. We continued to produce EksoVest and associated accessories for existing customers in 2022. EksoVest was discontinued at the end of 2022. EksoZeroG is a tool holder that can mount on an aerial lift platform or scaffolding. This effectively reduces the weight of heavy tools as felt by the operator. EksoZeroG has been sold primarily through rental companies into the construction market. EksoZeroG was discontinued in 2022. Market feedback continues to indicate a growing imperative among construction and manufacturing companies to drive adoption of improved safety and health practices. Furthermore, based on initial field-testing and market research, we believe that industrial exoskeletons have the potential to help prevent workforce injuries, improve productivity and over time reduce workers’ compensation and related costs. In the U.S. alone, our target manufacturing and construction verticals employ a total of 18.4 million workers (according to U.S. Bureau of Labor Statistics), many of whom can potentially benefit from our assistive technology. In addition, human augmentation technology is being viewed by senior managers of companies that have participated in field- testing as an opportunity to extend the careers of experienced and skilled workers while also changing the work environment to attract future workers to these careers. While we believe that the evidence clearly demonstrates that there is significant demand for human augmentation in industrial applications, adoption rates remain a challenge due to the nascent nature of the technology. That said, we believe that there is significant mid-to-long-term potential in the industrial markets, and accordingly, we will continue our product development efforts to expand our EksoWorks product offerings. Given the fragmented nature of the industrial market we believe that the best approach in this market involves collaboration with established strategic partners who can help us target applications tailored for specific use cases. We believe that leveraging our extensive exoskeleton expertise and intellectual property portfolio with the established channel and applying the expertise of one or more strategic partners will unlock the highest value for us and our stockholders. We continue to engage with multiple potential industrial partners, and plan to continue this approach going forward. Manufacturing and Service After Sales Service Maintenance and service support, primarily provided under the Ekso Care program for the EksoNR or extended warranty program for Ekso Indego, helps to maximize operational efficiency for our customers and reduces unplanned equipment downtime. We provide direct service for our customers’ devices at our facilities in San Rafael, California, Macedonia, Ohio and Ratingen, Germany. For some customers in EMEA and APAC, we utilize third-party service providers. Our team consists of service technicians, who perform repairs at our facilities or onsite as required and provide remote technical support, and customer care agents who resolve and troubleshoot issues that could inhibit optimal customer utilization. Beyond our extended warranty and Ekso Care service programs, we provide a fee-for-service option through which device repairs are fulfilled per quote on demand of the customer and as per our repair price list. Table of Contents 9 Manufacturing and Supply Chain We currently manufacture our EksoNR and EVO products at our facilities in San Rafael, California for worldwide sales. Our Ekso Indego Therapy and Ekso Indego Personal devices are manufactured at our facilities in Macedonia, Ohio. We currently run one shift per day at both facilities and believe we have the capacity to eventually run additional shifts should we deem it appropriate. In addition to our in-house manufacturing capabilities, we are in the process of adding contract manufacturing partners for both EksoNR and EVO. In 2022, we completed the process of transferring sufficient technology and know-how to build EksoNR at a domestic contract manufacturing partner. For the full year of 2022, contract manufacturing represented approximately 20% of our production output for EksoNR. We expect the share of contracted manufacturing production for EksoNR to expand further in 2023. Starting in 2022, we also began the process of adding external manufacturing capability for our EVO product line at a contract manufacturing partner located in Malaysia. We believe that manufacturing EVO at a contract manufacturing partner will help us to expand capacity, lower cost, and improve quality. We expect this process to be completed in the first half of 2023. For 2023, we expect the majority of our manufacturing output for EVO to be provided by this contract manufacturing partner. We purchase both custom and off-the-shelf components from a large number of suppliers and subject them to stringent quality specifications and processes. Whenever possible, we seek to secure dual source suppliers for our components. Some of the components necessary for the assembly of our products are currently provided to us by single-sourced suppliers (the only approved supply source for us among other sources). We purchase the majority of our components and major assemblies through purchase orders rather than long-term supply agreements and generally do not maintain finished goods in excess of our anticipated demand. We currently support our domestic contract manufacturing partner in the procurement of raw materials for EksoNR. Intellectual Property We have established an extensive intellectual property portfolio that includes various U.S. patents and patent applications. The table below provides a summary of U.S. patents by issuing status and ownership status as of December 31, 2022. Issuing Status License Status Issued Patents Pending Applications Licensed to the Company 9 3 Exclusively licensed to the Company 10 — Co-owned with a third party, exclusively licensed to the Company 5 — Co-owned with a third party 3 — Sole ownership by the Company 61 11 Total 88 14 Pending applications mean a complete application has been filed with the applicable patent authority and additional action is pending. Many of these applications have also been filed internationally as appropriate for their respective subject matter. As of December 31, 2022, 299 applications have issued or have been allowed as patents internationally. Our patent portfolio contains 334 cases that have issued or are in prosecution in 21 countries outside the U.S. Our patent portfolio includes product and method type claims, since the devices that we produce and the processes performed by those devices are patentable. Our patents encompass technologies relevant to our devices, including medical exoskeletons, commercial exoskeletons, actuators, and strength-enhancing exoskeletons. The earliest priority date of the portfolio reaches back to 2003, and new applications may continue to be filed from time-to-time. Licensors include the Regents of the University of California, or UC Berkeley, and Vanderbilt University. Table of Contents 10 The license with UC Berkeley consists of two agreements and one amendment to the agreement covering ten patent cases exclusively licensed to us, nine of which have issued and one of which remains in prosecution, or the UC Berkeley License Agreements. Inventions covered by a further three patent applications are co-owned by us and UC Berkeley, with no license agreement between us and UC Berkeley. As a result, UC Berkeley may license its rights in these patents to a third party. With respect to two of these co-owned patent applications, UC Berkeley has licensed their rights in the U.S. to an unrelated third party. The third patent application will need to be fully prosecuted before it can be determined which claims are exclusive to us (through a previous license) and which claims UC Berkeley may license to other entities. Pursuant to the UC Berkeley License Agreements, Ekso Bionics initially paid UC Berkeley consideration consisting of $5,000 in cash and 310,400 common shares of Ekso Bionics, and committed to pay a 1% royalty on sales, including sales generated by sublicenses. In addition, the UC Berkeley License Agreements call for minimum annual payments of $50,000. We do not pay royalties to UC Berkeley on products sold or to be resold to the U.S. government. As part of the HMC acquisition, Ekso acquired and assumed certain intangible assets including license agreements with Vanderbilt University. On October 15, 2012, PH entered a license agreement (“Exoskeleton License Agreement”) with Vanderbilt University and was granted exclusive license within the HMC field of use to specific licensed patents and licensed software by paying a non- refundable, non-creditable license issue fee and running royalties. Subsequently, PH entered three amendments with Vanderbilt University and was granted license to additional patents and software from 2014 to 2019 by paying license issue fee and running royalties. The royalties were set to be calculated at 6% of Net Sales for Licensed Patent Products (or a minimum of $250,000) and 3% of Net Sales for Licensed Software products. On March 1, 2022, Parker Hannifin Corporation entered a license agreement (“P-H Knee License Agreement”) with Vanderbilt University and was granted exclusive license to specific licensed patents, licensed software and copyrightable technical information by paying a non-refundable, non-creditable license issue fee and running royalties. Included in this agreement was the right to sublicense beginning in March 2024. We will pay Vanderbilt $100,000 as the second of two payments due April 30, 2023. In addition, royalties were set to be calculated at 3.75% of net sales of the licensed product. Beginning July 1, 2027, minimum annual royalties will be set at $75,000 (for the 12 month period through June 30, 2028) and $100,000 for each 12 month period thereafter. In addition to the aforementioned agreements, various other subsidized research and development agreements have been entered into with Vanderbilt covering specific work product as articulated in those documents. In some cases, as a result of government funding we receive, our patents have a government use license, granting the U.S. government a non-exclusive, non-transferable, irrevocable, paid-up license for use of the inventions for or on behalf of the U.S. government, as is typical in the case of government sponsored research. Under a license agreement with the developer of certain intellectual property related to mechanical balance and support arm technologies, which grants the Company an exclusive license with respect to the technology and patent rights for certain fields of use, the Company is required to pay the developer a single-digit royalty on net receipts, subject to a $50,000 annual minimum royalty requirement. The license agreement with this developer was terminated as of June 30, 2022. In addition, the Company entered into a license agreement in December of 2021 with a third party that develops technologies having utility in robotic exoskeletons from research and development activities associated with a specific set of government funded research projects. Commencing in January 2022, the Company assists with research and development activities in exchange for access to a worldwide, royalty free, transferable, sublicensable, exclusive license to design and market products that use or incorporate the jointly-developed technology within Ekso’s target market segments. Intellectual Property Out-Licensing In March 2018, we entered into a set of agreements with Daydo Co, Ltd., or Daydo, related to distribution and cross-licensing of the EksoVest. Under these agreements, Daydo has exclusive distribution rights for the EksoVest within Japan and rights to modify the EksoVest as needed to address the Japanese market in exchange for royalty payments to us. We also have rights to use any improvements made by Daydo. Daydo released its localized version of the EksoVest (called Task AR) in January of 2019. These agreements were terminated in 2022, resulting in the recognition of all deferred prepaid royalty revenue. Table of Contents 11

In June 2020, we entered into a non-exclusive license agreement with HAWE Hydraulik of Germany for rights to develop hydraulic pumps covered by a family of our patents. The agreement additionally includes an exclusivity option. We did not receive any royalty revenue from this license in the years ended December 31, 2022 and 2021. Competition The medical technology and industrial robotics industries are characterized by intense competition and rapid technological change. We believe that a number of other companies are developing competitive technology and devices for both the able- bodied and medical fields of use. In the medical field, we face competition from companies that are focused on technology for rehabilitation of patients suffering from stroke and related neurological disabilities as well as from companies that are focused on SCI. In stroke, Cyberdyne, ReWalk, Wandercraft, and ExoAtlet offer ambulatory exoskeletons for varying use cases within the rehabilitation markets where we operate. While not functionally equivalent, Hocoma, AlterG, Aretech and Reha Technology sell end-effector or treadmill-based gait therapies. Other companies that have announced plans to commercialize robotic exoskeletons include Bionik Laboratories and SuitX. The EksoNR device is the only FDA-cleared device for SCI, ABI (including stroke), and MS. Technologies developed by competitors in the areas of stroke rehabilitation and SCI represent therapeutic interventions with utility at varying points on the continuum of care. Clinically, the EksoNR is unique in its broad ability to mobilize pre- or even non-ambulatory patients using a full weight bearing, over ground, task-based platform. From a practice management perspective, the EksoNR is less expensive than many other systems, has a smaller footprint, has the ability to move around the hospital, and uses standard power requirements that make it easy to integrate into existing infrastructure. Other over-ground exoskeletons were initially designed for an individual to achieve ambulation reliant on the device. By contrast, the EksoNR’s design accommodates patients with complete paraplegia and additionally includes features that are optimized to assist therapists in helping patients with some motor ability learn to walk again in a clinical setting, treating several patients and indications in a single day. In the home and community ambulation use market ReWalk and Cyberdyne offer products that address similar use cases to Ekso Indego Personal in certain markets. We believe Ekso Indego Personal is a more robust and easier to use product than the competition. We also believe that given our strong position in rehabilitation clinics, we have a better channel to direct patients from clinical settings to our specific personal devices. Notwithstanding the foregoing, the most pressing challenges we face are not necessarily competitive technologies, but rather achieving rapid market awareness and adoption of this emerging technology while acclimating prospects to a fundamentally new paradigm in neuro-rehabilitation and mobility. In addition, it may be difficult for the rehabilitation department of a hospital or clinic to secure the funds to acquire Ekso devices in an environment where capital expenditures of this magnitude are not commonly incurred by those rehabilitation departments. In the industrial business, there are multiple competitors with shoulder devices including products from Ottobock, Levitate, Skel-ex, and others. While these products all address similar use cases in overhead work, we believe that EVO provides a better solution. In particular EVO provides i) optionally more support for larger users and those using heavy tools, ii) a wider range of shoulder motion free of obstructions from the device, especially when reaching directly overhead, iii) a more rugged, durable design, and iv) minimal contact points with the body to reduce heat and sweat generation. Exoskeleton technology remains in its infancy. As this field develops, we believe that we will face increased competition on the basis of product features, clinical outcomes, price, services and other factors. Our competitive position will depend on multiple, complex factors, including our ability to achieve market acceptance for our products, develop new products, implement production and marketing plans, secure regulatory approvals for products under development and protect our intellectual property. In some instances, competitors may also offer, or may attempt to develop, alternative therapies for disease states that may be delivered without a medical device. Table of Contents 12 Governmental Regulation and Product Approval U.S. Medical Device Regulation The U.S. government regulates the medical device industry through various agencies, including but not limited to the FDA, which administers the Federal Food, Drug and Cosmetic Act (FDCA). The design, testing, manufacturing, storage, labeling, distribution, advertising, and marketing of medical devices are subject to extensive regulation by federal, state, and local governmental authorities in the United States, including the FDA, and by similar agencies in other countries. Any medical device product that we develop must receive all requisite regulatory approvals or clearances, as the case may be, before it may be marketed in a particular country. Device development, marketing clearance and approval. The FDA classifies medical devices into one of three classes (Class I, II or III) based on the degree of risk the FDA determines to be associated with a device and the extent of control deemed necessary to ensure the device’s safety and effectiveness. Devices requiring fewer controls because they are deemed to pose lower risk are placed in Class I or II. Class I devices are deemed to pose the least risk and are subject only to general controls applicable to all devices, such as requirements for device labeling, premarket notification, and adherence to the FDA’s current good manufacturing practice requirements, as reflected in its Quality System Regulation (QSR). Class II devices are intermediate risk devices that are subject to general controls and may also be subject to special controls such as performance standards, product-specific guidance documents, special labeling requirements, patient registries or post-market surveillance. Class III devices are those for which insufficient information exists to assure safety and effectiveness solely through general or special controls, and include life- sustaining, life-supporting, or implantable devices, and devices not “substantially equivalent” to a device that is already legally marketed. Most Class I devices, and some Class II devices are exempted by regulation from the 510(k) clearance requirement and can be marketed without prior authorization from the FDA. Class I and Class II devices that have not been so exempted are eligible for marketing through the 510(k) clearance pathway. By contrast, devices placed in Class III generally require premarket approval (PMA), prior to commercial marketing. To obtain 510(k) clearance for a medical device, an applicant must submit a premarket notification application to the FDA demonstrating that the device is “substantially equivalent” to a predicate device, which is typically a Class II device that is legally marketed in the United States. A device is substantially equivalent to a predicate device if it has the same intended use and (i) the same technological characteristics, or (ii) has different technological characteristics and the information submitted demonstrates that the device is as safe and effective as a legally marketed device and does not raise different questions of safety or effectiveness. A showing of substantial equivalence sometimes, but not always, requires clinical data. Generally, the 510(k) clearance process can exceed 90 days and may extend to a year or more. After a device has received 510(k) clearance for a specific intended use, any modification that could significantly affect its safety or effectiveness, such as a significant change in the design, materials, method of manufacture or intended use, will require a new 510(k) clearance, or if the device as modified is not substantially equivalent to a legally marketed predicate device, a PMA. While the determination as to whether new authorization is needed is initially left to the manufacturer, the FDA may review this determination and evaluate the regulatory status of the modified product at any time and may request the manufacturer to cease marketing and recall the modified device until 510(k) clearance or PMA is obtained. The manufacturer may also be subject to significant regulatory fines or penalties. The second, more comprehensive, approval process applies to a new device that is not substantially equivalent to a predicate device or that is to be used in supporting or sustaining life or preventing impairment. These devices are normally Class III devices requiring PMA. The FDA will approve the PMA application if it finds there is reasonable assurance that the device is safe and effective for its intended use. The PMA process takes substantially longer than the 510(k) process, taking approximately one to two years or more for approval. In some instances, the FDA may find that a device is new and not substantially equivalent to a predicate device but is also not a high-risk device as is generally the case with Class III PMA devices. In these instances, the FDA may allow a device to be reclassified from Class III to Class I or II. The De Novo reclassification option is an alternate pathway to classify novel devices of low-to-moderate risk that had automatically been placed in Class III after receiving a “not substantially equivalent” (NSE) determination in response to a 510(k) notification. The FDA also allows a sponsor to submit a De Novo reclassification request to the FDA for novel low to moderate risk devices without first being required to submit a 510(k) application. These types of applications are referred to as “Evaluation of Automatic Class III Designation” or “De Novo requests.” In instances where a device is deemed not substantially equivalent to a Class II predicate device, the candidate device may be filed as a De Novo application which may lengthen regulatory decisions by the FDA. FDA review of a De Novo application may lead the FDA to identify the device as either a Class I or II device and subject to or exempt from 510(k) premarket notification. Clinical trials are generally required to support a PMA or De Novo reclassification application and are sometimes required for 510(k) clearance. Clinical trials generally require an investigational device exemption application (IDE), approved in advance Table of Contents 13 by the FDA for a specified number of patients and study sites, unless the product is deemed a non-significant risk device eligible for more abbreviated IDE requirements. Clinical trials are subject to extensive monitoring, recordkeeping and reporting requirements. Clinical trials must be conducted under the oversight of an institutional review board (IRB), for the relevant clinical trial sites and must comply with FDA regulations, including but not limited to those relating to good clinical practices. Conducting a clinical trial also requires obtaining the patients' informed consent in form and substance compliant with both FDA requirements and state and federal privacy and human subject protection regulations. The FDA or the IRB could suspend a clinical trial at any time for various reasons, including a belief that the risks to study subjects outweigh the anticipated benefits. Even if a trial is completed, the results of clinical testing may not adequately demonstrate the safety and efficacy of the device or may otherwise not be sufficient to obtain FDA approval to market the product in the U.S. To date, the EksoNR and EksoGT have been the subject of several clinical studies, some sponsored by us, as well as non-Ekso-sponsored independent studies conducted by rehabilitation institutions. Our current indications for use (IFU) clearance for ABI (including stroke), SCI, and MS. On April 1, 2016, we received clearance from the FDA to market our EksoGT robotic exoskeleton for use in the treatment of individuals with hemiplegia due to stroke, individuals with SCI at levels T4 to L5, and individuals with SCI at levels of T3 to C7 (ASIA D), in accordance with the device’s labeling. On July 19, 2016, we received clearance from the FDA to expand/clarify the indications and labeling to expressly include individuals with hemiplegia due to stroke who have upper extremity function of at least 4 out of 5 strength in at least one arm. On August 25, 2019, our EksoNR device was introduced with the same IFU as EksoGT. On June 15, 2020, we received clearance from FDA to expand the indications for use, or IFU, and labeling to expressly include individuals with ABI, including traumatic brain injury and stroke who have upper extremity function of at least 4 out of 5 strength in at least one arm. On June 9, 2022, we received further clearance from FDA to expand the IFU and labeling to expressly include individuals with multiple sclerosis (MS). After a device is placed on the market, numerous regulatory requirements apply. These include: • product listing and establishment registration, which helps facilitate FDA inspections and other regulatory action; • The quality system regulation, or QSR, which requires manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the manufacturing process; • labeling regulations and FDA prohibitions against the promotion of products for un-cleared, unapproved or off-label use or indication; • 510(k) clearance of product modifications that could significantly affect safety or efficacy or that would constitute a major change in intended use of one of our cleared devices; • medical device reporting regulations, which require that manufacturers comply with FDA requirements to report if their device may have caused or contributed to a death or serious injury, or has malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction of the device or a similar device were to recur; • post-approval restrictions or conditions, including post-approval study commitments; • post-market surveillance regulations, which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device; • the FDA's recall authority, whereby it can ask, or under certain conditions order, device manufacturers to recall from the market a product that is in violation of governing laws and regulations; • regulations pertaining to voluntary recalls; and • notices provision regarding corrections or removals. The FDA has broad post-market and regulatory enforcement powers. We are subject to unannounced inspections by the FDA to determine our compliance with the QSR and other regulations. Failure to comply with applicable regulatory requirements can result in enforcement action by the FDA, which may include any of the following sanctions: adverse publicity, warning letters, fines, injunctions, civil or criminal penalties, consent decrees, recall or seizure of our products, operating restrictions, partial suspension or total shutdown of production, refusing our request for 510(k) clearance or pre-market approval of new products, rescinding previously granted 510(k) clearances or withdrawing previously granted pre-market approvals. In the year ended December 31, 2022, there were no reports of an adverse event relating to our EksoNR or EksoGT devices reported to the FDA under the Manufacturer and User Facility Device Experience Database. Federal Anti-Kickback and Self-referral Laws The Federal Anti-Kickback Statute prohibits, among other things, the knowing and willful offer, payment, solicitation or receipt of any form of remuneration overtly or covertly, in cash or in kind, in return for, or to induce the: Table of Contents 14 • referral on an individual to a person for the furnishing or arranging for the furnishing of items or services reimbursable under Medicare, Medicaid, or other federal healthcare programs; or • purchase, lease, or order of, or the arrangement or recommendation of the purchasing, leasing, or ordering of any good, facility, item or service reimbursable under Medicare, Medicaid or other federal healthcare programs The Federal Anti-Kickback Statute applies to our arrangements with our United States sales representatives, customers and healthcare providers. Although we believe that we have structured such arrangements to comply with the Anti-Kickback Statute and other applicable laws, regulatory authorities may determine otherwise. Non-compliance with the Federal Anti-Kickback Statute can result in cancellation of our provider numbers and exclusion from Medicare, Medicaid or other federal healthcare programs, restrictions on our ability to operate in certain jurisdictions, as well as civil and criminal penalties, any of which could have an adverse effect on our business and results of operations. Federal law also includes the Physician Self-Referral Law, commonly known as the “Stark Law,” which prohibits a physician from referring a patient to an entity with which the physician (or an immediate family member of the physician) has a financial relationship, for the furnishing of certain designated health services for which payment may be made by Medicare or Medicaid, unless an exception applies. Violation of the Stark Law could result in denial of payment, disgorgement of reimbursements received under a non-compliant arrangement, civil penalties and fees, and exclusion from Medicare, Medicaid or other federal healthcare programs. Although we believe that we have structured our provider arrangements to comply with current Stark Law requirements, regulatory authorities may determine otherwise. Additionally, regulations issued for the Federal Anti-Kickback Statute and the Stark Law have undergone significant revisions, and it is reasonable to assume that revisions will occur in the future. While we have attempted to operate in compliance with these laws and regulations, our arrangements may ultimately be found to be not in compliance with applicable federal law. Federal False Claims Act The Federal False Claims Act provides, in part, that the federal government may bring a lawsuit against any person whom it believes has knowingly presented, or caused to be presented, a false or fraudulent request for payment to the federal government, or who has made a false statement or used a false record to get a claim approved. In addition, amendments in 1986 to the Federal False Claims Act have made it easier for private parties to bring “qui tam” or whistleblower lawsuits against companies. Although we believe that we are in compliance with the federal government’s laws and regulations, if we are found in violation of these laws, penalties of up to $0.025 million for each false claim, plus three times the amount of damages that the federal government sustained because of the act, can be assessed. Civil Monetary Penalties Law The Federal Civil Monetary Penalties Law grants authority to the U.S. Department of Health & Human Services Office of Inspector General (OIG) to seek civil monetary penalties (CMPs) against an individual or entity based on a wide variety of conduct including violations of the Anti-Kickback Statute, Stark Law, and False Claims Act. An entity that offers to or transfers remuneration to any individual eligible for benefits under Medicare or Medicaid that such entity knows or should know is likely to influence such individual to order or receive from a particular provider, practitioner, or supplier any Medicare or Medicaid payable item or service may be liable for CMPs. We sometimes offer customers various discounts and other financial incentives in connection with the sales of our products. While it is our intent to comply with all applicable laws, the federal government may find that our marketing activities violate the law. If we are found to be in non-compliance, we could be subject to CMPs of up to $0.112 million for each wrongful act, assessment of three times the amount claimed for each item or service and exclusion from Medicare, Medicaid and other federal healthcare programs. In addition, to the extent we are found to not be in compliance, we may be required to curtail or restructure our operations. Any penalties, damages, fines, exclusions, curtailment or restructuring of our operations could adversely affect our ability to operate our business and our financial results. State Fraud and Abuse Provisions Many states have also adopted some form of anti-kickback and self-referral laws and false claims act that may apply to DMEPOS suppliers regardless of the payor source. We believe that we are in compliance with such laws. Nevertheless, a determination of liability under such laws could result in fines and penalties and restrictions on our ability to operate in these jurisdictions. HIPAA Table of Contents 15

The Health Insurance Portability and Accountability Act of 1996, or HIPAA, established uniform standards governing the conduct of certain electronic healthcare transactions and protecting the security and privacy of individually identifiable health information maintained or transmitted by healthcare providers, health plans and healthcare clearinghouses, which are referred to as “covered entities.” Three standards have been promulgated under HIPAA’s regulations: the Standards for Privacy of Individually Identifiable Health Information, which restrict the use and disclosure of certain individually identifiable health information, the Standards for Electronic Transactions, which establish standards for common healthcare transactions, such as claims information, plan eligibility, payment information and the use of electronic signatures, and the Security Standards, which require covered entities to implement and maintain certain security measures to safeguard certain electronic health information, including the adoption of administrative, physical and technical safeguards to protect such information. In 2009, Congress passed the American Recovery and Reinvestment Act of 2009, or ARRA, which included sweeping changes to HIPAA, including an expansion of HIPAA’s privacy and security standards. ARRA includes the Health Information Technology for Economic and Clinical Health, or HITECH, which, among other things, made HIPAA’s privacy and security standards directly applicable to business associates of covered entities effective February 17, 2010. A business associate is a person or entity that performs certain functions or activities on behalf of a covered entity that involve the use or disclosure of protected health information in connection with recognized healthcare operations activities. As a result, business associates are now subject to significant civil and criminal penalties for failure to comply with applicable standards. Moreover, HITECH created a requirement to report certain breaches of unsecured, individually identifiable health information and imposes penalties on entities that fail to do so. HITECH also increased the civil and criminal penalties that may be imposed against covered entities, business associates and possibly other persons and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorney fees and costs associated with pursuing federal civil actions. The 2013 final HITECH omnibus rule modified the breach reporting standard in a manner that made more data security incidents qualify as reportable breaches. In addition to federal regulations issued under HIPAA, some states have enacted privacy and security statutes or regulations that, in some cases, are more stringent than those issued under HIPAA. In those cases, it may be necessary to modify our planned operations and procedures to comply with the more stringent state laws. If we fail to comply with applicable state laws and regulations, we could be subject to additional sanctions. Any liability from failure to comply with the requirements of HIPAA, HITECH or state privacy and security statutes or regulations could adversely affect our financial condition. The costs of complying with privacy and security related legal and regulatory requirements are burdensome and could have a material adverse effect on our results or operations. Patient Protection and Affordable Care Act In addition, there has been a recent trend of increased federal and state regulation of payments made to physicians and other healthcare providers. The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, among other things, imposed public reporting requirements on medical device manufacturers for payments or other transfers of value made by them to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members. The Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act enacted in 2018, extends the reporting and transparency requirements under the Physician Payments Sunshine Act to physician assistants, nurse practitioners and other mid-level practitioners, with reporting requirements going into effect in 2022 for payments made in 2021. Failure to submit required ownership and investment interest information may result in civil monetary penalties of up to an aggregate of $0.18 million per year (or up to an aggregate of $1.191 million per year for “knowing failures”), for all payments, transfers of value or ownership or investment interests that are not timely, accurately and completely reported in an annual submission. Certain states also mandate implementation of compliance programs, impose restrictions on device manufacturer marketing practices and/or require the tracking and reporting of gifts, compensation and other remuneration to physicians and other healthcare professionals. The Patient Protection and Affordable Care Act also requires healthcare providers to voluntarily report and return an identified Medicare or Medicaid overpayment within 60 days after identifying the overpayment. Failure to repay the overpayment within 60 days will result in the claim being considered a “false claim” and the healthcare provider will be subject to False Claims Act liability, and additional CMPs of $0.112 million for each item or service that is not reported and returned. Industrial and Medical Device Advertising Advertising and promotion of medical devices, in addition to being regulated by the FDA, are also regulated by the Federal Trade Commission (FTC) and by state regulatory and enforcement authorities. Recently, promotional activities for FDA- regulated products of other companies have been the subject of enforcement action brought under healthcare reimbursement laws and consumer protection statutes. If the FDA determines that promotional or training material related to a cleared device Table of Contents 16 constitutes the promotion of an un-cleared or unapproved use, the FDA could request that the promotional or training materials related to such device be modified or it could subject the manufacturer to regulatory or enforcement actions under the FDCA or other statutory authorities, such as law prohibiting false claims for reimbursement. In that event, our reputation could be damaged and adoption of the products would be impaired. The FTC regulates the advertising and promotion of products that are medical devices as well as non-medical medical products under the Federal Trade Commission Act (FTC Act). The FTC Act requires that an advertiser possess, at a minimum, a “reasonable basis” to substantiate all product claims before the claims are made, and competent and reliable scientific evidence to substantiate health and therapeutic claims. A lack of adequate substantiation may render such claims deceptive and/or misleading. The FTC may enforce compliance with the law in a variety of ways, both administratively and judicially, using compulsory process, cease and desist orders, and injunctions. FTC enforcement can result in orders requiring, among other things, limits on advertising, corrective advertising, customer redress, restitution, divestiture of assets, rescission of contracts, and such other relief as the agency deems necessary to protect the public. Violation of these orders could result in substantial financial or other penalties. Although we have not been the subject of any action by the FTC, no assurance can be given that the FTC will not question our advertising or other operations in the United States in the future. Any action in the future by the FTC could materially and adversely affect our ability to successfully market our products in the United States. In addition, under the federal Lanham Act and similar state laws, competitors and others can initiate litigation relating to advertising claims. Failure by us to comply with applicable regulations could result in substantial penalties, which could have a material adverse effect on our financial condition or results of operations and adversely affect our ability to successfully market our products in the United States. Foreign Medical Device Regulation In addition to regulations in the United States, we are subject to a variety of foreign regulations governing clinical trials and commercial sales and distribution of our products in foreign countries. Regardless of the FDA’s approval requirements for a particular product, we must obtain approval of a product by the comparable regulatory authorities of foreign countries before we can commence clinical trials or marketing of the product in those countries. The approval process varies from country to country, and the time may be longer or shorter than that required for FDA approval. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from country to country. In the EU, our products are subject to the medical device regulations of the various member states, which for many years were based on Directives of the European Commission. However, in May 2017, the EU adopted new, formal regulations to replace such Directives; specifically, the EU Medical Device Regulation (the “EU MDR”), which imposes stricter requirements for the marketing and sale of medical devices, including in the area of clinical evaluation requirements, quality systems and post- market surveillance. The EU regulations were adopted with staggered transitional periods that have since been updated. In January 2023, the European Commission endorsed a proposal to extend the original compliance dates for EU MDR, subject to approval by the European Parliament and European Council. The proposal would extend the current EU MDR transitional period deadline of May 2024 to 2027 or 2028, based upon the risk class of the device. Regulatory requirements in the United Kingdom (“UK”) are also changing as a result of Brexit (the UK’s withdrawal from the EU), and regulatory requirements in Switzerland are changing as a result of the country’s withdrawal from its Mutual Recognition Agreement with the EU Commission. Complying with the EU MDR and the evolving regulatory regimes in the UK and Switzerland requires modifications to our quality management systems, additional resources in certain functions and updates to technical files, among other changes. As of December 31, 2022, none of our products had yet been approved under EU MDR. The European Union requires that manufacturers of medical devices obtain the right to bear the “CE” conformity marking which designates compliance with existing directives and standards regulating the design, manufacture and distribution of medical devices in member countries of the European Union. In 2017, the European Union adopted the European Medical Device Regulation (Council Regulations 2017/745) which imposes stricter requirements for the marketing and sale of medical devices, including new clinical evaluation, quality system, and post-market surveillance requirements. The regulation had a three-year implementation period, with full application of the regulation occurring in May 2021 and replacing the pre-existing directives on medical devices in the European Union. Since May 2021, medical devices marketed in the European Union require certification according to these new requirements, except those devices with valid CE Marks, issued pursuant to the Medical Device Directive before May 2021, including our oxygen therapy products with CE Marks issued under the Medical Device Directive (MDD), may be placed on the market until May 2024. Only medical devices that comply with certain conformity requirements of the Medical Device Directive are currently allowed to be marketed within the European Union and our products will be required to comply with the EU MDR. New products that failed to be certified with the EU MDR by May 2021 may not be marketed or sold in the European Union. Similarly, existing products with CE Marks issued under the MDD may not be placed on the market in the European Union after May 2024. The extension of the existing certificates under the MDD or obtaining a new certificate under the MDR is required for continued marketing in the EU after May 18, 2022. Table of Contents 17 On November 5, 2021, we received notification from Health Canada that our EksoNR was reclassified from Class I to Class II, and requested that we reapply for registration under the Medical Device License (MDL) program. Until that license is established, we are restricted from marketing in that country. We are updating our quality system and applying for registration with the expectation that this matter will be resolved by early 2023. The policies of the FDA and foreign regulatory authorities may change, and additional government regulations may be enacted which could prevent or delay regulatory approval of our products and could also increase the cost of regulatory compliance. We cannot predict the likelihood, nature or extent of adverse governmental regulation that might arise from future legislative or administrative action, either in the U.S. or abroad. Human Capital Resources and Management As of March 23, 2023, we had 73 employees, including 60 full time employees and one part-time employee in the United States. Ten employees reside in Europe and two in Singapore. None of our employees are covered by a collective bargaining agreement and we consider our relationship with our employees to be good. We endeavor to maintain a workplace that is free from discrimination or harassment on the basis of color, race, sex, national origin, ethnicity, religion, age, disability, sexual orientation, gender identification or expression or any other status protected by applicable law. We conduct annual training to prevent harassment and discrimination and monitor employee conduct year- round, including by providing employees with access to an anonymous whistleblower hotline to report any violations. The basis for recruitment, hiring, development, training, compensation and advancement at the Company includes qualifications, performance, skills, and experience. We believe our employees are fairly compensated, without regard to gender, race and ethnicity, and routinely recognized for outstanding performance and are offered training and professional development opportunities. Our compensation program is designed to attract and retain talent. We continually assess and strive to enhance employee satisfaction and engagement. Corporate Information Our principal executive office is located at 101 Glacier Point, Suite A, San Rafael, California, 94901 and our telephone number is (510) 984-1761. We make available free of charge on or through our website our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and all amendments to those reports as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. Our internet address is www.eksobionics.com. This website address is intended to be an inactive, textual reference only; none of the material on this website is part of this Annual Report. Copies of our annual reports on Form 10-K will be furnished without charge to any person who submits a written request directed to the attention of our Secretary, at our offices located at 101 Glacier Point, Suite A, San Rafael, California, 94901. The SEC maintains an internet site (http://www.sec.gov) that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. Table of Contents 18 Item 1A. RISK FACTORS Investing in our common stock involves a high degree of risk. You should carefully consider the risks and uncertainties described below, together with all of the other information in this Annual Report on Form 10-K including the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and related notes, before making a decision to invest in our common stock. Our business, results of operations, financial condition or prospects could also be harmed by risks and uncertainties that are not presently known to us or that we currently believe are not material. If any of the risks actually occur, our business, results of operations and financial condition could be adversely affected. In that event, the market price of our common stock could decline, and you could lose all or part of your investment. Summary of Risk Factors Our business is subject to numerous risks and uncertainties that you should consider before investing in our company, as fully described below. The principal factors and uncertainties that make investing in our company risky include, among others: • The ongoing COVID-19 pandemic has adversely affected our financial condition and there is little future certainty. • The markets in which our products are sold are highly competitive and continue to develop. • We may not be able to reduce the cost to manufacture or service our products as planned. • If we or our third-party manufacturers are unable to produce our products at a satisfactory quality, in a timely manner, in sufficient quantities or at an acceptable cost, our business could be negatively impacted. • Shortages in the materials used to manufacture our products, as well as reductions in manufacturer capacity, could impact our future results. • Coverage policies and reimbursement levels of third-party payers may impact sales of our products. • The acquisition and integration of other companies, businesses, or technologies could result in operating difficulties, dilution, and other harmful consequences. • We may not be able to enhance our product offerings through our research and development efforts. • We have incurred significant losses to date and anticipate continuing to incur losses in the future, and we may not achieve or maintain profitability. • Our loan agreement imposes certain financial, and operational restrictions on us, limiting the discretion of our management in operating our business. • Protecting our intellectual proprietary rights can be costly, and our success in doing so is not certain. • If we fail to obtain or maintain necessary regulatory clearances or approvals for our medical device products, or if clearances or approvals for future products or modifications to existing products are delayed or not issued, our commercial operations would be harmed. • Modifications to our EksoNR and our future products may require new 510(k) clearances or premarket approvals, or may require us to cease marketing or recall the modified products until clearances are obtained • Our failure to meet strict post-market regulatory requirements with respect to our products could require us to pay fines, incur other costs or even close our facilities. • Our success depends on our management team, and on our ability to hire, train, retain, and motivate employees. Business and Operational Risks The ongoing COVID-19 pandemic has adversely affected our financial condition and there is little future certainty. The COVID-19 pandemic and related public health measures have materially affected how we and our customers are operating our businesses, and have materially affected our operating results; the duration and extent to which this will impact our future results remain uncertain. Although vaccine rollouts have improved the outlook of the global economy generally, renewed waves and new variants still pose concerns. Growth and investor confidence may be weakened by a variety of factors, including but not limited to, difficulties in containing the virus and related variants, limited availability of effective vaccines and other medical treatments, and stringent social distancing or lockdowns efforts. In the broader economy, supply chain disruption and resulting inflationary pressures, a global labor shortage, and the ebb and flow of COVID-19, including in specific geographies, are currently impacting the pace of global economic recovery and outlook, which could adversely affect our business. Table of Contents 19

Additionally, hospitals are facing significant financial pressure as supply chain constraints and inflation drive up operating costs and fiscal stimulus programs enacted during the COVID-19 pandemic wind down. As a consequence of the financial pressures and decreased profitability, some hospitals have indicated that they are lowering their capital investment plans and tightening their operational budgets. We are also subject to other risks applicable to businesses operating in the current environment. For example, our business insurance may not provide coverage against economic loss or claims specifically tied to COVID-19. A greater number of our employees are working remotely, which exposes us to a greater risk of cybersecurity breaches. The COVID-19 outbreak may also adversely impact our ability to make requisite filings under federal securities laws on a routine and timely basis. In addition, any deterioration in economic conditions due to the COVID-19 pandemic or any related market volatility may impact our ability to access the capital markets or ability to obtain financing on favorable terms or at all, which may affect our liquidity. The situation is constantly evolving, however, so the extent to which the COVID-19 outbreak will impact business and the economy is uncertain. Accordingly, consequences stemming from the ongoing COVID-19 pandemic could have a material adverse effect on our business, financial condition, results of operations and cash flows. The markets in which our products are sold are highly competitive and continue to develop. We face competition within the medical devices and industrial robotics markets on the basis of product features, clinical outcomes, price, services and other factors. Our competitive position will depend on multiple, complex factors, including our ability to achieve market acceptance for our products, develop new products, implement production and marketing plans, secure regulatory approvals for products under development and protect our intellectual property. Competitors may offer, or may attempt to develop, more efficacious, safer, cheaper, or more convenient alternatives to our products, including alternatives that could make the need for robotic exoskeletons obsolete. The entry into the market of manufacturers located in low-cost manufacturing locations may also create pricing pressure, particularly in developing markets. Our future success depends, among other things, upon our ability to compete effectively against current technology, as well as to respond effectively to technological advances, and upon our ability to successfully implement our marketing strategies and execute our research and development plan. If customers do not perceive our product offerings to be of value or to be easy and comfortable to use, we may not be able to attract and retain customers. If we are unable to successfully retain existing customers and attract new customers and achieve volume sales of our products, our business, prospects, financial condition and operating results will be materially and adversely affected. Furthermore, the markets for medical and industrial robotic exoskeletons are continuing to develop. We cannot be certain that the markets for robotic exoskeletons will continue to develop as we expect, or that robotic exoskeletons for medical or industrial use will achieve market widespread market acceptance. Additionally, the development of new or improved products, processes or technologies by other companies may render our products or proposed products less competitive or obsolete. The use of robotic devices is not universally accepted in the rehabilitation community and may never be. Current or future clinical trials and studies may not provide sufficient data that the rehabilitation community interprets to support the use of exoskeletons in rehabilitation. Any of these outcomes could materially and adversely affect our business, financial condition and operating results and prospects. We may not be able to reduce the cost to manufacture or service our products as planned. Our business plan assumes that exoskeletons can be manufactured more inexpensively than they are currently being manufactured. However, we have not yet found a way to significantly reduce the manufacturing cost of our products and doing so may prove more difficult than expected or even impossible. For example, if expectations for greater functionality of the products drive costs up as other factors drive costs down, the result may be that the overall cost of manufacturing the product stays the same or even increases. Likewise, we currently provide service and support of our products for our customers at a high standard (both in and out of warranty), and plan on continuing to do so. Our business plan also assumes that as we continue to improve our product, we achieve improved levels of product reliability and decreased service cost and frequency, which also may prove more difficult than expected. If we or our third-party manufacturers are unable to produce our products at a satisfactory quality, in a timely manner, in sufficient quantities or at an acceptable cost, our business could be negatively impacted. In order to reduce manufacturing costs, we intend to transition a significant amount of our manufacturing processes to third parties. Reliance on third parties to manufacture our products presents significant risks to us, including the potential that manufacturing costs may be higher than if we had kept manufacturing in house, as well as risks of reduced control over delivery schedules and product reliability, manufacturing deviations from internal and regulatory specifications, failure of a manufacturer to perform its obligations to us for technical, market or other reasons, misappropriation of our intellectual property, and other risks in meeting schedules and satisfying requirements of our customers. Table of Contents 20 We have not entered into any long-term manufacturing or supply agreements for any of our products, and we may need to enter into additional agreements for the commercial development, manufacturing and sale of our products. There can be no assurance that we can do so on favorable terms, if at all. Our products have been produced in quantities, and on timelines, sufficient to meet commercial demand and for us to satisfy our delivery schedules. However, our dependence upon others for the production of a portion of our products, or for a portion of the manufacturing process, may adversely affect our ability to satisfy demand, as well as to develop and commercialize new products, on a timely and competitive basis. If manufacturing capacity is reduced or eliminated at one or more of our third-party manufacturers’ facilities, we could have difficulties fulfilling our customer orders, which could adversely affect customer relationships, and our net revenues and results of operations could decline. Shortages in the materials used to manufacture our products, as well as reductions in manufacturer capacity, could impact our future results. Due to a variety of factors, including the COVID-19 pandemic, various materials we and the third-party manufacturers we rely on use to manufacture our products are currently, or may in the future, experience shortages and supply chain disruptions. For example, the global semiconductor industry has faced significant supply chain shortages and other disruptions as a result of increased demand, the inability of fabrication plants to produce sufficient quantities of chips to meet that demand, including as a result of government restrictions on staffing and facility operations in light of the COVID-19 pandemic, and other causes. Electronic components in general, battery cells, metals and plastics, all of which we use in our products, have, in the recent past, been are also in shorter supply compared to prior periods, and we are also experiencing longer lead times for manufacturing services such as machining and tool making. These and other factors are also causing plant shutdowns, reductions in capacity, delays and increased costs with our third-party manufacturers. Numerous factors, such as the ongoing pandemic or further trade tensions between the United States and China, may prolong or deepen these challenges. Our operating results may be negatively impacted if global supply chains of semiconductors and other important commodities in short supply do not normalize. Coverage policies and reimbursement levels of third-party payers may impact sales of our products. To the extent that the adoption of our products by our customers is dependent in the future on their ability to obtain adequate reimbursement for treatments provided using our product from third-party payers, the coverage policies and reimbursement levels of these third-party payers may impact the decisions of healthcare providers and facilities to purchase our products or the prices they would be willing to pay for those products. Reimbursement rates could also affect the acceptance rates of new technologies. We have no control over these factors. We will experience long and variable sales cycles. The EksoNR and Ekso Indego have a lengthy sale and purchase order cycle because it is a major capital expenditure item and generally requires the approval of senior management at purchasing institutions, which may contribute to substantial fluctuations in our quarterly operating results. International sales of our products are subject to factors outside of our control. Our business currently depends in part on our activities in the EMEA, APAC, and other foreign markets. Our international activities are subject to a number of risks inherent in selling and operating abroad, including failure of local laws to provide the same degree of protection against infringement of our intellectual property rights; protectionist laws and business practices that favor local competitors, which could slow our growth in international markets; the expense of establishing facilities and operations in new foreign markets; building an organization capable of supporting geographically dispersed operations; challenges caused by distance, language and cultural differences; challenges caused by differences in legal regulations, markets, and customer preferences, which may limit our ability to adapt our products or succeed in other regions; multiple, conflicting, and changing laws and regulations, including complications due to unexpected changes in regulatory requirements, foreign laws, tax schemes, international import and export legislation, trading and investment policies, exchange controls and tariff and other trade barriers; foreign tax consequences; fluctuations in currency exchange rates and foreign currency translation adjustments; foreign exchange controls that might prevent us from repatriating income earned outside the United States; imposition of public sector controls; differing payer reimbursement regimes, governmental payers or patient self-pay systems and price controls; political, economic and social instability; and restrictions on the export or import of technology. We may not be able to enhance our product offerings through our research and development efforts. In order to increase our sales and our market share in the exoskeleton market, we continue to invest in our research and development efforts and product offerings in response to the evolving demands of people with lower extremity impairment, other medical conditions and healthcare providers, as well as competitive technologies. We may decide to invest our business Table of Contents 21 development resources in partnerships, licensing agreements, business acquisition and other ways that will provide us new product offerings without significant research and development activities. We may not be successful in developing, obtaining regulatory approval for, or marketing our currently proposed products, or our approved products for additional indications, products proposed to be created in the future or products that will be available for us through business acquisitions. In addition, notwithstanding our market research efforts, our future products may not be accepted by consumers, their caregivers, healthcare providers or third-party payors who reimburse consumers for our products. The success of any proposed product offerings will depend on numerous factors, including our ability to: • identify the product features that people with lower extremity impairment, their caregivers, and healthcare providers are seeking in a medical device that restores mobility and successfully incorporate those features into our products; • identify the product features that people with lower extremity impairment or other similar indications require while the products are used at home as well as what items are valuable to the clinics that provide them rehabilitation; • develop and introduce proposed products in sufficient quantities and in a timely manner; • adequately protect our intellectual property and avoid infringing upon the intellectual property rights of third- parties; • demonstrate the safety, efficacy, and health benefits of proposed products; and • obtain the necessary regulatory clearances and approvals for proposed products. If we fail to generate demand by developing products that incorporate features desired by consumers, their caregivers or healthcare providers, or if we do not obtain regulatory clearance or approval for proposed products in time to meet market demand, we may fail to generate sales sufficient to achieve or maintain profitability. We have in the past experienced, and we may in the future experience, delays in various phases of product development, including during research and development, manufacturing, limited release testing, marketing, and customer education efforts. Such delays could cause customers to delay or forgo purchases of our products, or to purchase our competitors’ products. Even if we are able to successfully develop proposed products when anticipated, these products may not produce sales in excess of the costs of development, and they may be quickly rendered obsolete by changing consumer preferences or the introduction by our competitors of products embodying new technologies or features. We may never complete the development of any of our proposed products or product improvements into marketable products. We do not know when or whether we will successfully complete the development of the planned development-stage or next generation exoskeletal technologies, or any other proposed, developmental, or contemplated product for any of our target markets. We continue to seek to improve our technologies before we are able to produce a commercially viable product. Failure to improve on any of our technologies could delay or prevent their successful development for any of our target markets. Developing any technology into a marketable product is a risky, time consuming and expensive process. You should anticipate that we will encounter setbacks, discrepancies requiring time consuming and costly redesigns and changes and that there is the possibility of outright failure. We may not meet our product development, manufacturing, regulatory, commercialization and other milestones. We have historically relied, and in the future may rely, on sales of our EksoNR, Ekso Indego Therapy and Ekso Indego Personal for a significant portion of our revenue. We currently rely, and in the future will rely, on sales of our EksoNR, Ekso Indego Therapy and Ekso Indego Personal for a large portion of our revenue. These products are relatively new, and market acceptance and adoption depends on educating people with lower extremity impairment, physical therapists and other clinicians as to the distinct features, ease-of-use, improved quality of life and other benefits when compared to alternative therapies. These products may not be perceived to have sufficient potential benefits compared with their alternatives. In addition, physical therapists and other clinicians may be slow to change their treatment practices because of perceived liability risks arising from the use of new products. Accordingly, physical therapists and other clinicians may not recommend these products until there is sufficient evidence to convince them to alter the treatment methods they typically recommend. Such evidence may include endorsements from prominent healthcare providers or other key leaders in the lower extremity impairment and neurological impairment communities attesting to the effectiveness of these products in providing identifiable immediate and long-term quality of life benefits, and the publication of peer-reviewed clinical studies demonstrating their value. Any factors that negatively impact sales of these products would adversely affect our business, financial condition and operating results. Table of Contents 22 We rely on independent distributors for the sale and marketing of our products in certain geographies. In non-German-speaking European countries, other EMEA countries and Central and South American countries, we rely on independent distributors to distribute and assist us with the marketing and sale of our products. While we expect that the percentage of our sales generated from independent distributors will decrease over time as we continue to focus our resources on achieving reimbursement within our direct markets in non-German-speaking European countries, other EMEA countries and Central and South American countries, we believe that some percentage of our sales will continue to be generated by independent distributors in the future. Additionally, since closing the HMC Acquisition, we have relied on other independent distributors for the sale and marketing of our Ekso Indego Therapy and Ekso Indego Personal These distributors are our principal customers, and revenue growth will depend in large part on our success in establishing and maintaining this sales and distribution channel. However, there can be no assurance that our distributors will be successful in selling our products to end users, or will focus adequate resources on selling them, and they may not continue to purchase or market our products for a number of reasons. If any of our key independent distributors were to cease to distribute our products, our sales could be adversely affected. In such a situation, we may need to seek alternative independent distributors or increase our reliance on our other independent distributors or our direct sales representatives, which may not prevent our sales from being adversely affected. Further, to the extent our newly acquired distribution channels have contractual or other limitations that may impact the economies of scale we would otherwise receive as a result of the HMC Acquisition, our business and prospects may be adversely affected. Our success depends on our management team, and on our ability to hire, train, retain, and motivate employees. Our success depends on our management team and on our ability to identify, hire, train and retain highly qualified managerial, technical and sales and marketing personnel. Any significant leadership change and accompanying senior management transition, such as the recent change in our chief executive officer, and the hiring of other new leaders in key roles, involves inherent risk and any failure to ensure a smooth transition could hinder our strategic planning, execution and future performance. In addition, as we introduce new products or services, we will need to hire additional personnel. Currently, competition for personnel with the required knowledge, skill and experiences is intense, particularly in the San Francisco Bay area, where we are headquartered, and we may not be able to attract, assimilate or retain such personnel. The inability to attract and retain the necessary managerial, technical and sales and marketing personnel could have a material adverse effect on our business, results of operations and financial condition. The acquisition and integration of other companies, businesses, or technologies could result in operating difficulties, dilution, and other harmful consequences. We may selectively pursue strategic acquisitions, any of which could be material to our business, operating results, and financial condition, like the HMC Acquisition. Future acquisitions could divert management’s time and focus from operating our business. In addition, integrating an acquired company, business or technology is risky and may result in unforeseen operating difficulties and expenditures associated with integrating employees from the acquired company into our organization and integrating each company’s accounting, management information, human resources and other administrative systems to permit effective management. For example, in connection with the HMC Acquisition, we expanded our manufacturing footprint to Ohio through a temporary lease at Parker Hannifin Corporation's Ohio facilities that is generally set to expire in December 2023, it continues to require internal resources and may ultimately be unsuccessful. While we believe this expansion will be beneficial for our business and that we will be able to find a more permanent location in Ohio. The anticipated benefits of future acquisitions may not materialize, including our ability to expand our product offerings as a result of overlap in the addressable market for our existing products and the addressable market for products we may acquire. Future acquisitions or dispositions could result in potentially dilutive issuances of our equity securities, the incurrence of debt, contingent liabilities or amortization expenses, or write-offs of goodwill, any of which could harm our financial condition. Future acquisitions may also require us to obtain additional financing, which may not be available on favorable terms or at all. If we fail to manage the complex and lengthy reimbursement process, our business and operating results could be adversely affected. We accept assignment of insurance benefits from customers and, in a majority of cases, invoice and collect payments directly from Medicare, private payors and Medicaid, as well as direct from patients under co-insurance provisions. We also distribute our products through the VA hospitals. The VA maintains its policy of covering the cost of our devices for qualifying veterans. Our financial condition and results of operations may be affected by the VA coverage policy and the healthcare industry’s reimbursement process, which is complex and can involve lengthy delays between the time that a product is delivered to the consumer and the time that the reimbursement amounts are settled. Depending on the payor, we may be required to obtain certain payor-specific documentation from physicians and other healthcare providers before submitting claims for reimbursement. Certain payors have filing deadlines and they will not pay claims submitted after such time. We are also subject to extensive pre-payment and post-payment audits by governmental and private payors that could result in material delays, Table of Contents 23

refunds of monies received or denials of claims submitted for payment under such third-party payor programs and contracts. We cannot ensure that we will be able to continue to effectively manage the process which would adversely affect our business, financial condition and results of operations. Shutdowns of the U.S. federal government could materially impair our business and financial condition. Development of our product candidates or regulatory approval may be delayed for reasons beyond our control. For example, in 2018 and 2019 the U.S. government shut down several times and certain regulatory agencies, such as the FDA and the SEC, had to furlough critical FDA, SEC, and other government employees and stop critical activities. If a prolonged government shutdown or budget sequestration occurs, it could significantly impact the ability of the FDA to timely review and process our regulatory submissions, which could have a material adverse effect on our business. Further, in our operations as a public company, future government shutdowns could impact our ability to access the public markets, such as through the declaration of effectiveness of registration statements and obtain necessary capital in order to properly capitalize and continue our operations. Financial & Accounting Risks We have incurred significant losses to date and anticipate continuing to incur losses in the future, and we may not achieve or maintain profitability. We have thus far been largely dependent on capital raised through the sale of equity securities in various public and private offerings, and we have incurred losses in each fiscal year since our incorporation in 2005. Our net losses were $15.1 million and $9.8 million for the years ended December 31, 2022 and 2021, respectively (with gains on revaluation of warrant liabilities from a decrease in our common stock purchase price resulting in a $1.3 million reduction to our net loss for 2022 and gain on revaluation of warrant liabilities from an decrease in our common stock purchase price resulting in a $4.0 million reduction to our net loss in 2021). As of December 31, 2022 and 2021, we had an accumulated deficit of $223.9 million and $208.9 million, respectively. The operation of our business and our growth efforts will require significant cash outlays and advance capital equipment expenditures and commitments. We believe we have sufficient resources to operate for the foreseeable future based upon our current cash resources, the recent rate of using cash for operations and investment, and assuming modest increases in current revenue offset by incremental increases in expenses related to increased sales and marketing and research and development, and a potential increase in subscription activity from our medical device business. However, unless we are able to generate significant revenues from sales and subscriptions of our products, we will not be able to achieve or maintain profitability in the near future or at all, and we will remain largely dependent on capital raised from past and future financings to implement our business plan, support our operations and service our debt obligations. Our lack of profitability may depress our stock price, and if we are unable to become profitable, we may be required to reduce the scope of our business development activities, which could harm our business plans, financial condition and operating results, or to cease our operations entirely. Our loan agreement imposes certain financial, and operational restrictions on us, limiting the discretion of our management in operating our business. Our loan agreement with Pacific Western Bank, which we entered into in August 2020 (the "PWB Loan Agreement"), contains, subject to certain carve-outs, various restrictive covenants that limit our management's discretion in operating our business. In particular, these instruments limit our ability to, among other things incur additional debt, grant liens on assets, sell or acquire assets outside the ordinary course of business, pay dividends and make certain fundamental business changes. Our obligations, which become due in August 2023, are also secured by a security interest in all of our assets, exclusive of intellectual property. As a result, we may need to use our capital resources to repay the PWB Loan in order to undertake certain financing or strategic transactions. We may be unable to generate sufficient cash flow to service our debt obligations and operate our business. As described in Note 10 to the consolidated financial statements, we have material near-term indebtedness due to the PWB Loan Agreement and the $5 million unsecured, subordinated promissory note (the “Promissory Note”) we delivered to Parker Hannifin Corporation in connection with the HMC Acquisition. Servicing our debt requires a significant amount of cash. While we anticipate that we will have adequate cash resources to fund our operations and satisfy our debt obligations, our ability to generate sufficient cash depends on numerous factors beyond our control and our business may not generate sufficient cash flow from operating activities. Our ability to make payments on, and refinance, our debt and fund planned capital expenditures will depend on our ability to generate cash in the future. To some Table of Contents 24 extent, this is subject to general economic, financial, competitive, legislative, regulatory and other factors that are beyond our control, including rising interest rates. We cannot assure our business will generate sufficient cash flow from operations, or future borrowings will be available to us in an amount sufficient to fund our liquidity needs. If our cash flows and capital resources are insufficient to service our indebtedness, we may be forced to reduce or delay capital expenditures, sell assets, seek additional capital or restructure or refinance our indebtedness. These alternative measures may not be successful and may not permit us to meet our scheduled debt service obligations. Our ability to restructure or refinance our debt will depend on the condition of the capital markets and our financial condition at such time. Any refinancing of our debt could be at higher interest rates and may require us to comply with more onerous covenants, which could further restrict our business operations. We may not have sufficient funds to meet our future capital requirements. As of December 31, 2022, we had $20.5 million in cash. While we believe we have sufficient cash to fund our operations for at least twelve months from the issuing date of this Annual Report, we cannot provide assurance that these funds will be sufficient to meet our future capital requirements. Our management will have broad discretion in the application of these capital resources, including for working capital and other general corporate purposes, which may include repayment of debt, acquisitions and other business opportunities. The amounts and timing of our use of proceeds will vary depending on a number of factors, including the amount of cash generated or used by our operations, and the rate of growth, if any, of our business, as well as our debt repayment obligations. In addition, we may use our cash on hand to pursue acquisitions of other businesses, products or technologies that are complementary to our business, joint ventures and licensing arrangements, and other strategic transactions and business opportunities. Our ability to secure financing and the cost of raising such capital are dependent on numerous factors, including general economic and capital markets conditions, credit availability from lenders, investor confidence and the existence of regulatory and tax incentives that are conducive to raising capital. Uncertainty in the financial markets has caused banks and financial institutions to decrease the amount of capital available for lending and has significantly increased the risk premium of such borrowings. In addition, such turmoil and uncertainty has significantly limited the ability of companies to raise funds through the sale of equity or debt securities. If we are unable to raise additional funds, we may need to delay, modify or abandon some or all of our business plans or cease operations. If we raise funds through the issuance of debt, the amount of any indebtedness that we may raise in the future may be substantial, and we may be required to secure such indebtedness with our assets and may have substantial interest expenses. If we default on any future indebtedness, our lenders could declare all outstanding principal and interest to be due and payable and our secured lenders may foreclose on the facilities securing such indebtedness. The incurrence of indebtedness could require us to meet financial and operating covenants, which could place limits on our operations and ability to raise additional capital, decrease our liquidity and increase the amount of cash flow required to service our debt. If we raise funds through the issuance of equity securities, such issuance could result in dilution to our stockholders and the newly issued securities may have rights senior to those of the holders of our common stock. We may not be able to leverage our cost structure or achieve better margins. Due to the early stage customer adoption of our products, our current sales and marketing, research and development, and general and administrative expenses are each a higher percentage of sales than they will need to be for us to reach profitability. While we do expect these expenses to grow as our business grows, we also expect these expenses to decline as a percentage of revenues over time. If we are unable to leverage these costs and grow revenues at a greater pace than these operating expenses as we expect, we will not be able to achieve viable operating margins and profitability. We could fail to maintain effective internal control over our financial reporting. Section 404 of the Sarbanes-Oxley Act of 2002 requires us to include in our annual reports on Form 10-K and quarterly reports on Form 10-Q an assessment by management of the effectiveness of our internal control over financial reporting. While we believe that the policies, processes and procedures we have put in place will be sufficient to render our internal controls over financial reporting effective, our initiatives may not prove successful. If so, management may not be able to conclude that our internal control over financial reporting is effective. This could result in a loss of investor confidence in the reliability of our financial statements, which in turn could negatively affect the price of our common stock. In addition, we must perform system and process evaluation and testing of our internal control over financial reporting to allow management to report on the effectiveness of our internal control over financial reporting, as required by Section 404. Our compliance with Section 404 may require that we incur substantial accounting expense and expend significant management efforts. Intellectual Property Risks Protecting our intellectual proprietary rights can be costly, and our success in doing so is not certain. Table of Contents 25 Our long-term success largely depends on our ability to market technologically competitive products. Failure to protect or to obtain, maintain or extend adequate patent and other intellectual property rights could have a material adverse impact on our competitive advantage and impair our business. Our issued patents may not be sufficient to protect our intellectual property and our patent applications may not result in issued patents. Even if our patent applications issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors from competing with us or otherwise provide us with any competitive advantage. Our competitors may be able to circumvent our patents by developing similar or alternative technologies or products in a non-infringing manner or may challenge the validity of our patents. Our attempts to prevent third parties from circumventing our intellectual property and other rights ultimately may be unsuccessful. We may also fail to take the required actions or pay the necessary fees to maintain any of our patents that issue. Furthermore, we have not filed applications for all of our inventions internationally and may not be able to prevent third parties from using our proprietary technologies or may lose access to technologies critical to our products in other countries. These include, in some cases, countries in which we are currently selling products and countries in which we intend to sell products in the future. Intellectual property litigation and infringement claims could cause us to incur significant expenses or prevent us from selling certain of our products. The industries in which we operate, including, in particular, the medical device industry, are characterized by extensive intellectual property litigation and, from time to time, we might be the subject of claims by third parties of potential infringement or misappropriation. Regardless of outcome, such claims are expensive to defend and divert the time and effort of our management and operating personnel from other business issues. A successful claim or claims of patent or other intellectual property infringement against us could result in our payment of significant monetary damages and/or royalty payments or negatively impact our ability to sell current or future products in the affected category and could have a material adverse effect on our business, cash flows, financial condition or results of operations. Because competition in our industry is intense, competitors may infringe or otherwise violate our issued patents, patents of our licensors or other intellectual property. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time-consuming. Any claims we assert against perceived infringers could provoke these parties to assert counterclaims against us alleging that we infringe their patents. In addition, in a patent infringement proceeding, a court may decide that a patent of ours is invalid or unenforceable, in whole or in part, construe the patent’s claims narrowly, or refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation proceeding could put one or more of our patents at risk of being invalidated or interpreted narrowly. We may also elect to enter into license agreements in order to settle patent infringement claims or to resolve disputes prior to litigation, and any such license agreements may require us to pay royalties and other fees that could be significant. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure. Some of the patents and patent applications in the intellectual property portfolio are not within our complete control, which could reduce the value of such patents. Some of our U.S. patents (which have associated international patents and applications) are co-owned by UC Berkeley. UC Berkeley has exclusively licensed its rights under many of these patents to us, but we do not have an exclusive license to UC Berkeley’s rights under three of these patents. UC Berkeley has licensed their U.S. rights in two of these three co-owned patents to an unrelated third-party. The third patent is a continuation-in-part of a patent that UC Berkeley has licensed to us. Under the terms of the relevant license agreement between us and UC Berkeley, we have exclusive rights to any claims that are fully supported by the specification in the parent application. However, any claims that are not based on the specification in the parent application are co-owned by UC Berkeley and us, and UC Berkeley’s rights in respect of such claims are not exclusively licensed to us. There is no assurance that we will be able to obtain a license to UC Berkeley’s rights in any such claims on commercially reasonable terms or at all, and UC Berkeley may choose to license its rights to third parties instead of us. In addition, in connection with our acquisition of certain assets from Equipois, we assumed the rights and obligations of Equipois with respect to certain patents and patent applications under an in-license of intellectual property from a third-party and subject to an out-license of that intellectual property to an unrelated third-party for use in a particular field. We do not have complete control over the prosecution of these patent applications. As well, the license of intellectual property rights under these patents to third parties could reduce the value of our patent portfolio and limit any income or license fees that we might receive if we were to attempt to transfer or license our rights under any of our co-owned or licensed patents. If we fail to comply with our obligations in the agreements under which we license intellectual property rights from third- Table of Contents 26 parties or otherwise experience disruptions to our business relationships with our licensors, we could lose intellectual property rights that are important to our business. We are a party to two exclusive license agreements and one amendment to the license agreement with UC Berkeley, covering ten patents exclusively licensed to us. In addition, in connection with our acquisition of certain assets from Equipois, we assumed the rights and obligations of Equipois with respect to certain patents and patent applications under an in-license of intellectual property from a third-party and subject to an out-license of that intellectual property to an unrelated third-party for use in a particular field. We may also need to obtain additional licenses from others to advance our research and development activities or allow the commercialization of our devices or any other devices we may identify and pursue. Our license agreements with UC Berkeley and the rights and obligations that we assumed in connection with the Equipois acquisition impose various development, diligence, commercialization, and other obligations on us, and any future license agreements may impose similar or other obligations on us. For example, under our license agreements with UC Berkeley we are required to submit a commercialization plan with performance milestones and progress report to UC Berkeley, and must satisfy specified minimum annual royalty payment obligations. In spite of our efforts, our licensors might conclude that we have materially breached our obligations under such license agreements and might therefore terminate the license agreements, thereby removing or limiting our ability to develop and commercialize products and technology covered by these license agreements. If our license agreements with UC Berkeley are terminated, or if our agreements granting us intellectual property rights in connection with the Equipois acquisition or any future agreements granting us material intellectual property rights are terminated or impeded in a material way, competitors or other third parties would have the freedom to seek regulatory approval of, and to market, products that may be identical or functionally similar to our devices and we may be required to cease our development and commercialization of such devices. Any of the foregoing could have a material adverse effect on our competitive position, business, financial conditions, results of operations and prospects. Moreover, disputes may arise between us and our counterparties regarding intellectual property subject to a licensing agreement, including the scope of rights granted under the license agreement and other interpretation-related issues; the extent to which our devices, technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement; the sublicensing of patent and other rights under our collaborative research and development relationships; our diligence obligations under the license agreement and what activities satisfy those diligence obligations; the ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our partners; and the priority of invention of patented or patentable technology. In addition, certain provisions in our license agreement with UC Berkeley may be susceptible to multiple interpretations. The resolution of any contract interpretation disagreement that may arise could narrow what we believe to be the scope of our rights to the relevant intellectual property or technology, or increase what we believe to be our financial or other obligations under the agreement, either of which could have a material adverse effect on our business, financial condition, results of operations and prospects. Moreover, if disputes over intellectual property that we have licensed prevent or impair our ability to maintain our current licensing arrangements on commercially acceptable terms, we may be unable to successfully develop and commercialize the affected devices, which could have a material adverse effect on our business, financial conditions, results of operations and prospects. Patent terms may be inadequate to protect our competitive position on our devices for an adequate amount of time. Patents have a limited lifespan. In the United States, if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its earliest U.S. non-provisional filing date. Various extensions may be available, but the life of a patent, and the protection it affords, is limited. Even if patents covering our devices are obtained, once the patent life has expired, we may be open to competition from competitive products. Given the amount of time required for the development, testing and regulatory review of new devices, patents protecting such devices might expire before or shortly after such devices are commercialized. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours. Legal and Regulatory Compliance Risks If we fail to obtain or maintain necessary regulatory clearances or approvals for our medical device products, or if clearances or approvals for future products or modifications to existing products are delayed or not issued, our commercial operations would be harmed. Our EksoGT, EksoNR, EksoUE, and Ekso Indego products are medical devices and are regulated by the FDA, the European Union and other governmental authorities both inside and outside of the United States. These agencies enforce laws and regulations that govern the development, testing, clinical trials, manufacturing, labeling, advertising, marketing and distribution, recordkeeping, recalls and field safety corrective actions, and market surveillance of our medical products. Our failure to comply with these complex laws and regulations could have a material adverse effect on our business, results of operations, financial condition and cash flows. Table of Contents 27

In the United States, before we can market a new medical device, or a new use of, new claim for or significant modification to an existing product, we must first receive either clearance under Section 510(k) of the FDCA or approval of a PMA application from the FDA, unless an exemption applies. Both the PMA and the 510(k) clearance process can be expensive, lengthy and uncertain. The FDA’s 510(k) clearance process may take anywhere from several months to over a year. The process of obtaining a PMA is much more costly and uncertain than the 510(k) clearance process and generally takes from one to three years, or even longer, from the time the application is filed with the FDA. In addition, PMA generally requires the performance of one or more clinical trials. The FDA also has substantial discretion in the medical device review process. Despite the time, effort and cost, we cannot assure you that any particular device will be approved or cleared by the FDA. Any delay or failure to obtain necessary regulatory approvals could harm our business. Failure can occur at any stage, and we could encounter problems that cause us to repeat or perform additional development, standardized testing, pre-clinical studies and clinical trials. Any delay or failure to obtain necessary regulatory approvals could harm our business. The FDA or other non-U.S. regulatory authorities can delay, limit or deny clearance or approval of a medical device candidate for many reasons, including a medical device candidate may not be deemed to be substantially equivalent to a device lawfully marketed either as a grandfathered device or one that was cleared through the 510(k) premarket notification process; a medical device candidate may not be deemed to be substantially equivalent to a device lawfully marketed either as a grandfathered device or one that was cleared through the 510(k) premarket notification process; a medical device candidate may not be deemed to be in conformance with applicable standards and regulations; FDA or other regulatory officials may not find the data from pre-clinical studies and clinical trials or other product testing date to be sufficient; other non-U.S. regulatory authorities may not approve our processes or facilities or those of any of our third-party manufacturers, thereby restricting export; or the FDA or other non-U.S. regulatory authorities may change clearance or approval policies or adopt new regulations. Even after regulatory clearance or approval has been granted, a cleared or approved product and its manufacturer are subject to extensive regulatory requirements relating to manufacturing, labeling, packaging, adverse event reporting, storage, advertising and promotion, recordkeeping, and recalls and field safety corrective actions of the product. If we fail to comply with the regulatory requirements of the FDA or other non-U.S. regulatory authorities, or if previously unknown problems with our products or manufacturing processes are discovered, we could be subject to administrative or judicially imposed sanctions, including restrictions on the products, manufacturers or manufacturing process; adverse publicity; adverse inspectional observations (Form 483), warning letters, non-warning letters incorporating inspectional observations; consent decrees; civil or criminal penalties or fines; injunctions; product seizures, detentions or import bans; voluntary or mandatory product recalls and publicity requirements; suspension or withdrawal of regulatory clearances or approvals; total or partial suspension of production; imposition of restrictions on operations, including costly new manufacturing requirements; refusal to clear or approve pending applications or premarket notifications; and import and export restrictions. If imposed on us, any of these sanctions could have a material adverse effect on our reputation, business, results of operations and financial condition. Modifications to our EksoNR and our future products may require new 510(k) clearances or premarket approvals, or may require us to cease marketing or recall the modified products until clearances are obtained On April 4, 2016, we received clearance from the FDA to market our EksoGT robotic exoskeleton for use in the treatment of individuals with hemiplegia due to stroke, individuals with SCI at levels T4 to L5, and individuals with SCI at levels of T3 to C7 (ASIA D), in accordance with the device’s labeling. On July 19, 2016, we received clearance from the FDA to expand/ clarify the indications and labeling to expressly include individuals with hemiplegia due to stroke who have upper extremity function of at least four-fifths in only one arm. Our prior cleared indications for use statement required that individuals with hemiplegia due to stroke have upper extremity function of at least four-fifths in both arms. On June 9, 2022, we received further clearance from FDA to expand the IFU and labeling to expressly include individuals with MS. An element of our strategy is to continue to upgrade our robotic exoskeleton platform to incorporate new software and hardware enhancements. Any modification to a 510(k)-cleared device, including our EksoGT, that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, design, or manufacture, requires a new 510(k) clearance or, possibly, a PMA. The FDA requires every manufacturer to make this determination in the first instance based on the final guidance document issued by the FDA in October 2017 addressing when to submit a new 510(k) application due to modifications to 510(k)-cleared devices and a separate guidance document on when to submit a new 510(k) application due to software changes to 510(k)-cleared devices. Although largely aligned with the FDA’s longstanding guidance document issued in 1997, the 2017 guidance includes targeted changes intended to provide additional clarity on when a new 510(k) application is needed. The FDA may review our determinations regarding whether new clearances or approvals are necessary, and may not agree with our decisions. If the FDA disagrees with our determinations for any future changes, or prior changes to previously marketed products, as the case may be, we may be required to cease marketing or to recall the modified products until we obtain clearance or approval, and we may be subject to significant regulatory fines or penalties. Table of Contents 28 We may introduce new products with enhanced features and extended capabilities from time to time. The products may be subject to various regulatory processes, and we may need to obtain and maintain regulatory approvals in order to sell our new products. If a potential purchaser of our products believes that we plan to introduce a new product in the near future or if a potential purchaser is located in a country where a new product that we have introduced has not yet received regulatory approval, planned purchases may be deferred or delayed. As a result, new product introductions may adversely impact our financial results. We must obtain certain regulatory approvals in the EU, which could be costly and time-consuming and subject us to unanticipated delays or prevent us from marketing certain devices. In the EU, we are required to comply with the EU MDR and obtain CE Certificates of Conformity in order to affix the CE Mark and market medical devices. As of December 31, 2022, none of our products had yet been approved under the EU MDR. We are currently in the process of obtaining Parker’s CE Certificates of Conformity in order to affix the CE Mark to the products we acquired in the HMC Acquisition, including Ekso Indego Personal and Ekso Indego Personal. Any delay in, or failure to receive or maintain the CE Mark as required under the EU MDR for the products acquired in the HMC Acquisition may prevent us from selling those products within the EU. In addition, changes in regulatory policy for the approval or CE marking of a medical device during the period of product development and regulatory agency review or notified body review of each submitted new application may cause delays or rejections. In January 2023, the European Commission endorsed a proposal to extend the original compliance dates for the EU MDR, subject to approval by the European Parliament and European Council. The proposal would extend the current MDR transitional period deadline of May 2024 to 2027 or 2028, based upon the risk class of the device. Failure to comply with the EU MDR requirements by the MDR transitional period deadline would prevent us from generating revenue from sales of our products in the EU, which could adversely affect our business, results of operations and financial condition. Our failure to meet strict post-market regulatory requirements with respect to our products could require us to pay fines, incur other costs or even close our facilities. We are required to comply with the FDA’s Quality System Regulation, or QSR, which is a complex regulatory scheme that covers the procedures and documentation of the design, testing, production, process controls, quality assurance, labeling, packaging, handling, storage, distribution, installation, servicing and shipping of our marketed products. These regulatory requirements may significantly increase our production costs and may even prevent us from making our products in amounts sufficient to meet market demand. If we change our approved manufacturing process, the FDA may need to review the process before it may be used. The FDA enforces the QSR through periodic announced and unannounced inspections of manufacturing facilities. Failure to comply with regulatory requirements such as QSR may result in changes to labeling, restrictions on such products or manufacturing processes, withdrawal of the products from the market, voluntary or mandatory recalls, a requirement to repair, replace or refund the cost of any medical device we manufacture or distribute, fines, suspension of regulatory approvals, product seizures, injunctions or the imposition of civil or criminal penalties which would adversely affect our business, operating results and prospects. Federal, state and non-U.S. regulations regarding the manufacture and sale of medical devices are subject to future changes. The complexity, timeframes and costs associated with obtaining marketing clearances are unknown. Although we cannot predict the impact, if any, these changes might have on our business, the impact could be material. We may be subject to fines, penalties or injunctions if we are determined to be promoting the use of our products for unapproved or “off-label” uses. Any cleared or approved product may be promoted only for its indicated uses and our promotional materials must comply with FDA and other applicable laws and regulations. We believe that the specific use for which our products are marketed fall within the scope of the indications for use that have been cleared by the FDA. However, if the FDA determines that our promotional materials or training constitutes promotion of an unapproved use, it could request that we modify our promotional materials or subject us to regulatory or enforcement actions, including the issuance of an untitled letter, a warning letter, injunction, seizure, civil fine and criminal penalties. It is also possible that other federal, state or foreign enforcement authorities might take action if they consider our promotional or training materials to constitute promotion of an unapproved use, which could result in significant fines or penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement. In that event, our reputation could be damaged and adoption of the products would be impaired. We may be subject to adverse medical device reporting obligations, voluntary corrective actions or agency enforcement actions. Table of Contents 29 Under the FDA’s medical device reporting or MDR regulations, we are required to report to the FDA any incident in which our product may have caused or contributed to a death or serious injury or in which our product malfunctioned and, if the malfunction were to recur, would likely cause or contribute to death or serious injury. For example, we have been informed of a limited number of events with respect to our EksoNR or EksoGT devices that have been determined to be reportable pursuant to the MDR regulations. In each case, the required MDR report was filed with the FDA. In addition, all manufacturers bringing medical devices to market in the European Economic Area are legally bound to report any incident that led or might have led to the death or serious deterioration in the state of health of a patient, user or other person, and which the manufacturer’s device is suspected to have caused, to the competent authority in whose jurisdiction the incident occurred. In such case, the manufacturer must file an initial report with the relevant competent authority, which would be followed by further evaluation or investigation of the incident and a final report indicating whether further action is required. The events described above that were reported to the FDA were also reported to the relevant EU regulatory authorities. We are also required to follow detailed recordkeeping requirements for all Company-initiated medical device corrections and removals, and to report such corrective and removal actions to the FDA if they are carried out in response to a risk to health and have not otherwise been reported under the MDR regulations. The FDA and similar foreign governmental authorities also have the authority to require the recall of commercialized products in the event of material deficiencies or defects in design, labeling or manufacture of a product or in the event that a product poses an unacceptable risk to health. Depending on the corrective action we take to redress a product’s deficiencies or defects, the FDA may require, or we may decide, that we will need to obtain new approvals or clearances for the device before we may market or distribute the corrected device. Seeking such approvals or clearances may delay our ability to replace the recalled devices in a timely manner. Moreover, if we do not adequately address problems associated with our devices, we may face additional regulatory enforcement action, including adverse publicity, FDA warning letters, product seizure, injunctions, administrative penalties, or civil or criminal fines. We may also be required to bear other costs or take other actions that may have a negative impact on our sales as well as face significant adverse publicity or regulatory consequences, which could harm our business, including our ability to market our products in the future. Any adverse event involving our products could result in future voluntary corrective actions, such as recalls or customer notifications, or agency action, such as inspection or enforcement action. Recalls of our products, or agency actions relating to our failure to comply with our reporting or recordkeeping obligations, could harm our reputation and financial results. Failure to comply with anti-kickback and fraud regulations could result in substantial penalties and changes in our business operations. Although we do not provide healthcare services, submit claims for third-party reimbursement, or receive payments directly from Medicare, Medicaid or other third-party payers for our products, we are subject to healthcare fraud and abuse regulation and enforcement by federal, state and foreign governments, which could significantly impact our business. These laws may constrain the business and financial arrangements and relationships through which we conduct our operations, including how we research, market, sell and distribute any product for which we have obtained regulatory approval, or for which we obtain regulatory approval in the future. The principal U.S. federal laws implicated include, but are not limited to, those that prohibit, among other things, (i) filing, or causing to be filed, false or improper claims for federal payment, known as the false claims laws, (ii) payment, solicitation or receipt of unlawful inducements, directly or indirectly, for the referral of business reimbursable under federally-funded health care programs, known as the anti-kickback laws, and (iii) health care service providers from seeking reimbursement for providing certain services to a patient who was referred by a physician who has certain types of direct or indirect financial relationships with the service provider, known as the Stark law. Many states have similar laws that apply to reimbursement by state Medicaid and other government funded programs as well as in some cases to all payers. Efforts to ensure that our business arrangements will comply with applicable healthcare laws and regulations will involve substantial costs. We are subject to the risk that a person or government could allege we have engaged in fraud or other misconduct, even if none occurred. It is possible that governmental and enforcement authorities will conclude that our business practices do not comply with current or future statutes, regulations or case law interpreting applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us now or in the future, we may be subject to penalties, including civil and criminal penalties, damages, fines, disgorgement, exclusion from governmental health care programs, additional integrity oversight and reporting obligations, contractual damages, reputational harm and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our financial results. Changes in law or regulation could make it more difficult and costly for us to manufacture, market and distribute our products or obtain or maintain regulatory approval of new or modified products. Table of Contents 30 From time to time, legislation is drafted and introduced in Congress that could significantly change the statutory provisions governing the regulatory approval, manufacture and marketing of regulated devices. In addition, FDA regulations and guidance are often revised or reinterpreted by the FDA in ways that may significantly affect our business and our products. Any new regulations or revisions or reinterpretations of existing regulations may impose additional costs or lengthen review times of future products. In addition, FDA regulations and guidance are often revised or reinterpreted by the agency in ways that may significantly affect our business and our products. Elections could result in significant changes in, and uncertainty with respect to, legislation, regulation and government policy that could significantly impact our business and the health care industry. It is impossible to predict whether legislative changes will be enacted or FDA regulations, guidance or interpretations changed, and what the impact of such changes, if any, may be. Any change in the laws or regulations that govern the clearance and approval processes relating to our current and future products could make it more difficult and costly to obtain clearance or approval for new products, or to produce, market, and distribute existing products. Significant delays in receiving clearance or approval, or the failure to receive clearance or approval, for any new products would have an adverse effect on our ability to expand our business. Healthcare changes in the United States and other countries, including recently enacted legislation reforming the U.S. healthcare system, could have a negative impact on our future operating results. In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory proposals to change the health care system in ways that could affect our ability to sell our products profitably. For example, in 2010, the Patient Protection and Affordable Care Act, or ACA, was enacted into law. The legislation seeks to reform the United States healthcare system. It is far-reaching and is intended to expand access to health insurance coverage, improve quality and reduce costs over time. We expect the law will have a significant impact upon various aspects of our business operations. The ACA reduces Medicare and Medicaid payments to hospitals, clinical laboratories and pharmaceutical companies, and could otherwise reduce the volume of medical procedures. These factors, in turn, could result in reduced demand for our products and increased downward pricing pressure. It is also possible that the ACA will result in lower reimbursements. While the ACA is intended to expand health insurance coverage to uninsured persons in the United States, the impact of any overall increase in access to healthcare on sales of our products remains uncertain. Since its enactment, there have been numerous judicial, administrative, executive, and legislative challenges to certain aspects of the ACA, and we expect there will be additional challenges in the future. As a result, there have been delays in the implementation of, and action taken to repeal or replace, certain aspects of the ACA. Most recently, under President Biden, the Department of Justice dropped support of two Supreme Court cases challenging the ACA in addition to a case before the U.S. Court of Appeals for the Fifth Circuit. We cannot predict the impact that such actions against the ACA or other health care reform under the Biden administration will have on our business, and there is uncertainty as to what healthcare programs and regulations may be implemented or changed at the federal and/or state level in the United States, or the effect of any future legislation or regulation. However, it is possible that such initiatives could have an adverse effect on our ability to obtain approval and/or successfully commercialize products in the United States in the future. For example, any changes that reduce, or impede the ability to obtain, reimbursement for the type of products we intend to commercialize in the United States (or our products more specifically, if approved) could adversely affect our business plan to introduce our products in the United States. Other legislative changes have been proposed and adopted in the United States since the ACA was enacted. For example, in August 2011, the Budget Control Act of 2011, among other things, created measures for spending reductions by Congress. A Joint Select Committee on Deficit Reduction, tasked with recommending a targeted deficit reduction of at least $1.2 trillion for the years 2012 through 2021, was unable to reach required goals, thereby triggering the legislation’s automatic reduction to several government programs. This includes aggregate reductions of Medicare payments to providers of up to 2% per fiscal year, which went into effect in April 2013 and will remain in effect through 2030 unless additional Congressional action is taken. Further, there has been heightened governmental scrutiny in recent years over the manner in which manufacturers set prices for their marketed products and the cost of prescription drugs to consumers and government healthcare programs, which have resulted in several recent Congressional inquiries and proposed and enacted bills designed to, among other things, reduce the cost of prescription drugs, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for products. In addition, the United States government, state legislatures, and foreign governments have shown significant interest in implementing cost containment programs, including price-controls, restrictions on reimbursement and requirements for substitution of generic products for branded prescription drugs to limit the growth of government paid health care costs. For example, the United States government has passed legislation requiring pharmaceutical manufacturers to provide rebates and discounts to certain entities and governmental payors to participate in federal healthcare programs. Further, Congress and the current administration Table of Contents 31

have each indicated that it will continue to seek new legislative and/or administrative measures to control drug costs, and the current administration recently released a “Blueprint”, or plan, to reduce the cost of drugs. The current administration’s Blueprint contains certain measures that the U.S. Department of Health and Human Services is already working to implement. Individual states in the United States have also been increasingly passing legislation and implementing regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. Additional changes may affect our business, including those governing enrollment in federal healthcare programs, reimbursement changes, fraud and abuse enforcement, and expansion of new programs, such as Medicare payment for performance initiatives. These initiatives, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and in additional downward pressure on the price that we receive for any approved product. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms could result in reduced demand for our product candidates or additional pricing pressures and may prevent us from being able to generate revenue, attain profitability, or commercialize our products. Finally, future elections in the United States could result in significant changes in, and uncertainty with respect to, legislation, regulation, implementation of Medicare and/or Medicaid, and government policy that could significantly impact our business and the healthcare industry. The President and the executive branch of the federal government have a significant impact on the implementation of the provisions of the ACA, and the current or future administrations could make changes impacting the implementation and enforcement of the ACA, which could harm our business, operating results and financial condition. If we are slow or unable to adapt to any such changes, our business, operating results and financial condition could be adversely affected. Failure to comply with the Federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, the Health Information Technology for Economic and Clinical Health Act, or HITECH Act, and implementing regulations could result in significant penalties. Numerous federal and state laws and regulations, including HIPAA and the HITECH Act, govern the collection, dissemination, security, use and confidentiality of patient-identifiable health information. HIPAA and the HITECH Act require us to comply with standards for the use and disclosure of such protected health information within our company and with third parties. The Privacy Standards and Security Standards under HIPAA establish a set of basic national privacy and security standards for the protection of individually identifiable health information by health plans, healthcare clearinghouses and certain healthcare providers, referred to as covered entities, and the business associates with whom such covered entities contract for services. Notably, whereas HIPAA previously directly regulated only these covered entities, the HITECH Act, which was signed into law in 2009, makes certain of HIPAA’s privacy and security standards directly applicable to covered entities’ business associates. Both covered entities and business associates are subject to significant civil and criminal penalties for failure to comply with the Privacy Standards and Security Standards under HIPAA. HIPAA requires healthcare providers like us to develop and maintain policies and procedures with respect to protected health information that is used or disclosed, including the adoption of administrative, physical and technical safeguards to protect such information from unauthorized disclosure. The HITECH Act expanded the notification requirement for breaches of patient- identifiable health information, restricts certain disclosures and sales of patient-identifiable health information and provides a tiered system for civil monetary penalties for HIPAA violations. The HITECH Act also increased the civil and criminal penalties that may be imposed against covered entities, business associates and possibly other persons and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorney fees and costs associated with pursuing federal civil actions. The 2013 final HITECH omnibus rule modified the breach reporting standard in a manner that made more data security incidents qualify as reportable breaches. Additionally, certain states have adopted comparable privacy and security laws and regulations, some of which may be more stringent than HIPAA. If we are determined to be out of compliance with existing or new laws and regulations related to patient health information, we could be subject to criminal or civil sanctions. New health information standards, whether implemented pursuant to HIPAA, the HITECH Act, congressional action or otherwise, could have a significant effect on the manner in which we handle healthcare related data and communicate with payors, and the cost of complying with these standards could be significant. Table of Contents 32 Any liability from a failure to comply with the requirements of HIPAA or the HITECH Act could adversely affect our results of operations and financial condition. The costs of complying with privacy and security related legal and regulatory requirements are burdensome and could have a material adverse effect on our results of operations. Regulations requiring the use of “standard transactions” for healthcare services issued under HIPAA may negatively affect our profitability and cash flows. Pursuant to HIPAA, final regulations have been implemented to improve the efficiency and effectiveness of the healthcare system by facilitating the electronic exchange of information in certain financial and administrative transactions while protecting the privacy and security of the information exchanged. The HIPAA transaction standards are complex, and subject to differences in interpretation by third-party payors. For instance, some third-party payors may interpret the standards to require us to provide certain types of information, including demographic information not usually provided to us by physicians. As a result of inconsistent application of transaction standards by third-party payors or our inability to obtain certain billing information not usually provided to us by physicians, we could face increased costs and complexity, a temporary disruption in accounts receivable and ongoing reductions in reimbursements and net revenue. Changes and updates to HIPAA transaction standards could prove technically difficult, time- consuming or expensive to implement, all of which could harm our business. Regulatory requirements under Proposition 65 could adversely affect our business. We are subject to California’s Proposition 65, or Prop 65, which requires a specific warning on any product that contains a substance listed by the State of California as having been found to cause cancer or birth defects, unless the level of such substance in the product is below a safe harbor level. Prop 65 required that all businesses must be in compliance by August 30, 2018 with new regulations that require modifications to product warnings and for businesses to coordinate with upstream vendors or downstream customers for the 800+ regulated chemicals in consumer products and assess whether new occupational exposure warnings need to be posited in California facilities. We have taken steps to add warning labels to our products packaged in California and manufactured after August 30, 2018. Although we cannot predict the ultimate impact of these requirements, they could reduce overall consumption of our products or leave consumers with the perception (whether or not valid) that our products do not meet their health and wellness needs, all of which could adversely affect our business, financial condition and results of operations. We are subject to evolving laws, regulations, and other obligations related to privacy, data protection, and information security, and our actual or perceived failure to comply with such obligations could harm our reputation, subject us to significant fines and liability or otherwise adversely affect our business, financial condition, and operating results. The regulatory frameworks for privacy, data protection, and information security issues worldwide are rapidly evolving and likely to remain uncertain for the foreseeable future. The U.S. federal and various state, local, and foreign government bodies and agencies have adopted or are considering adopting laws and regulations governing the collection, distribution, use, disclosure, storage, security, and other processing of personal information. For example, California adopted the California Consumer Privacy Act (CCPA), which became effective in January 2020. The CCPA establishes a privacy framework for covered businesses, including an expansive definition of personal information and data privacy rights for California residents. The CCPA includes a framework with potentially severe statutory damages and private rights of action. Additionally, a new privacy law, the California Privacy Rights Act (CPRA), was approved by California voters in the November 2020 election and went into effect on January 1, 2023. The CPRA significantly modifies the CCPA, potentially resulting in further uncertainty. Other states have begun to propose and enact similar laws. The U.S. federal government also is contemplating federal privacy legislation. Compliance with these laws and regulations is a rigorous and time-intensive process, and we may be required to put in place additional mechanisms to comply with such laws and regulations. The collection and use of health data and other personal data is governed in the EU by the General Data Protection Regulation (GDPR), which imposes substantial obligations upon companies and rights for individuals, and by certain EU member state- level legislation. Failure to comply with the GDPR may result in fines up to the greater of €20,000,000 or 4% of the total worldwide annual turnover of the preceding financial year. The UK has implemented legislation similar to the GDPR, referred to as the UK GDPR, which provides for fines of up to the greater of £17.5 million or 4% of global turnover. Many other jurisdictions globally are considering or have enacted legislation providing for local storage of data or otherwise imposing privacy, data protection, and data security obligations in connection with the collection, use, and other processing of personal data. As a general matter, compliance with laws, regulations, contractual obligations, and other actual and asserted obligations, Table of Contents 33 such as industry standards, and any rules or guidance from self-regulatory organizations, relating to privacy, data protection, and data security that apply, or are asserted to apply, to our operations may result in substantial costs and may necessitate changes to our policies and practices, which may compromise our growth strategy, adversely affect our ability to acquire customers, and otherwise adversely affect our business, results of operations, and financial condition. With laws, regulations, and other obligations relating to privacy, data protection, and information security imposing new and relatively burdensome obligations, and with substantial uncertainty over the interpretation and application of these and other obligations, we may face challenges in addressing their requirements and making necessary changes to our policies and practices. We also may incur significant costs and expenses in an effort to do so. Additionally, if third parties we work with, such as contractors or service providers, violate applicable laws or regulations or our policies, such violations may also put our data at risk and could in turn have an adverse effect on our business. Any failure or perceived failure by us or our contractors or service providers to comply with our applicable policies or notices, our contractual or other obligations to third parties, or any of our other actual or asserted legal obligations relating to privacy or data protection, may result in governmental investigations or enforcement actions, litigation, claims, and other proceedings, harm our reputation, and could result in significant liability. Any such event may adversely affect our business, operating results, and financial condition. We are subject to cybersecurity risks to our systems, infrastructure, and technology, and data processed by us or third-party vendors. Our business and operations involve the collection, storage, transmission, and other processing of personal data and certain other sensitive and proprietary data. Numerous organizations have disclosed breaches of their information security systems and other information security incidents, some of which have involved sophisticated and highly targeted attacks. We have been and may in the future be a target for cybersecurity attacks designed to disrupt our operations or to attempt to gain access to our systems, data processed or maintained in our business, trade secrets, or other proprietary information or financial resources. Many of our personnel work remotely all or part of the time, which increases certain security risks. In addition, the risk of state- supported and geopolitical-related cybersecurity attacks is believed to be heightened in connection with the war in Ukraine and any related political or economic responses and counter-responses. We are at risk for interruptions, outages, and breaches of our operational systems, including business, financial, accounting, product development, data processing or production processes, as well as our security systems, in-product software and technology, and customer data. We use third parties to process some data on our behalf, and they face similar security risks. Because techniques used to obtain unauthorized access to or to sabotage information systems change frequently and may not be known until launched against a target, we and the third parties on which we rely may be unable to anticipate or prevent these attacks, react in a timely manner or implement adequate preventive measures, and we may face delays in our detection or remediation of, or other responses to, security breaches and other privacy-and security-related incidents. Such incidents could materially disrupt our systems, result in loss of intellectual property and misappropriation of trade secrets or other proprietary or competitively sensitive information, compromise the confidentiality, security, and integrity of our information, including employees’ personal information, and information of customers or others, jeopardize the security of our facilities, or affect the performance of our products. The loss, corruption, or unavailability of clinical trial data from completed or future clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the impacted data. Certain efforts may be state-sponsored or supported by significant financial and technological resources, making them even more difficult to detect, remediate and otherwise respond to. Although we have implemented and are in the process of implementing additional systems and processes that are designed to protect our data and systems within our control, prevent data loss, and prevent other security breaches and security incidents, these measures cannot guarantee security. The systems and infrastructure used in our business may be vulnerable to cyberattacks or security breaches or incidents, and third parties may be able to access data, including personal data and other sensitive and proprietary data or other sensitive and proprietary data, or such data otherwise may be subject to unauthorized use, disclosure, unavailability, modification, or other processing. Employee error, malfeasance or other errors in the storage, use or transmission of any of these types of data could result in an actual or perceived privacy or security breach or other security incident. Any security breach or security incident impacting our systems or infrastructure, or data we or third parties on which we rely maintain or otherwise process, or any outages or other disruptions to systems used in our business, could interrupt our operations and result in the loss of or improper access to, or acquisition or disclosure of, data or a loss of intellectual property protection. Any such breach or incident, or the perception it has occurred, also may harm our reputation and competitive position, harm our product development and regulatory approval efforts, reduce demand for our products, damage our relationships with customers, partners, collaborators or others, and result in claims, demands, litigation, regulatory investigations and proceedings and significant legal, regulatory and financial exposure. Any such event may adversely affect Table of Contents 34 our business, operating results, and financial condition. We expect to incur significant costs in an effort to detect and prevent privacy and security breaches and other privacy- and security-related incidents, and may face increased costs and requirements to expend substantial resources in the event of an actual or perceived privacy or security breach or other incident. While we maintain insurance that may cover certain liabilities in connection with certain disruptions, security breaches, and incidents, our insurance policies may not be adequate to compensate us for the potential losses arising from any disruption in or, failure or security breach or incident of or impacting our systems or third-party systems where information important to our operations or product development is stored or processed. In addition, such insurance may not be available to us in the future on economically reasonable terms, or at all. Further, our insurance may not cover all claims made against us and could have high deductibles in any event, and defending a suit, regardless of its merit, could be costly and divert management attention. Product Liability Risks Our products may become subject to voluntary or involuntary recall. The FDA and similar foreign governmental authorities have the authority to require the recall of commercialized products in the event of material deficiencies or defects in design or manufacture or in the event that a product poses an unacceptable risk to health. In addition, manufacturers may, under their own initiative, recall a product if any material deficiency in a device is found. A government-mandated or voluntary recall by us could occur as a result of an unacceptable risk to health, component failures, manufacturing errors, design or labeling defects or other deficiencies and issues. To date, we have initiated only one field action in which we voluntarily accelerated our maintenance schedule based on field usage. When a medical human exoskeleton is used by a paralyzed individual to walk, the individual relies completely on the exoskeleton to hold them upright. There are many exoskeleton components that, if they were to fail catastrophically, could cause a fall resulting in severe injury or death of the patient. Certain of our competitors have reported injuries caused by the malfunction of human exoskeleton devices (in at least one case to the FDA). Injuries caused by the malfunction or misuse of human exoskeleton devices, even where such malfunction or misuse occurs with respect to one of our competitor’s products, could cause regulatory agencies to implement more conservative regulations on the medical human exoskeleton industry, which could significantly increase our operating costs. Similarly, when an industrial exoskeleton is used by a healthy individual - for example to operate heavy machinery overhead - malfunction of the device at an inopportune moment could result in severe injury or death of the person using the device. Such occurrences could result in regulatory action on the part of OSHA or its foreign counterparts. Any future recalls of any of our products could divert managerial and financial resources, impair our ability to manufacture our products in a cost-effective and timely manner, and have an adverse effect on our reputation, results of operations and financial condition. In some circumstances, such adverse events could also cause delays in new product approvals. We may also be required to bear other costs or take other actions that may have a negative impact on our future sales and our ability to generate profits. In addition, personal injuries relating to the use of our products could also result in product liability claims being brought against us. Any product liability claim brought against us, with or without merit, could result in substantial damages, be costly and time-consuming to defend and could increase our insurance rates or prevent us from securing insurance coverage in the future. Our product liability insurance may not adequately cover potential claims or recalls. The testing, manufacture, marketing and sale of medical devices and industrial products entail the inherent risk of liability claims or product recalls. Although we maintain product liability insurance, the coverage is subject to deductibles and limitations, and may not be adequate to cover future claims. A successful product liability claim or product recall could inhibit or prevent the successful commercialization of our products, cause a significant financial burden on us, or both, which in either case could have a material adverse effect on our business and financial condition. Warranty claims and our accelerated maintenance program results in additional operating costs to us. Sales of our EksoNR and Ekso Indego products generally include a one-year warranty for parts and services in the United States and a two-year warranty in EMEA. We also generally provide customers with an option to purchase an extended warranty for up to an additional three to four years. The costs associated with such warranties, including any warranty-related legal proceedings, could have a material adverse effect on our results of operations, cash flows and liquidity. As we enhance our product and in an effort to build our brand and drive adoption, we have elected to incur increased service expenses related to an accelerated maintenance program, field corrections and the implementation of technological improvements developed subsequent to many of our units being placed into service, sometimes outside of its warranty and contractual obligations. Continuation of these activities could have a material adverse effect on our results of operations, cash flows and liquidity. Table of Contents 35

Risks Related to Ownership of Common Stock You may be diluted from future issuances of our equity securities, including from compensatory equity awards, exercise of outstanding warrants, or issuances of securities in financing or strategic transactions, and such issuances, or perception that such issuances may occur, could depress the market price of our common stock. Future operating or business decisions may cause dilution to our stockholders. For example, we may sell equity securities or issue securities exercisable or convertible into shares of our common stock in connection with strategic transactions or for financing purposes, including under an At The Market Offering Agreement we entered into in October 2020 with H.C. Wainwright & Co., LLC ("Wainwright") or otherwise through our “shelf” registration statement on Form S-3 (File No. 333-239203). Through Fenruary 28, 2023, we have $6.7 million available for future offerings under our current prospectus for our “at the market offering”. We may also make equity grants under our Amended and Restated 2014 Incentive Plan (the “Incentive Plan”) and our Employee Stock Purchase Plan. You may also be subject to dilution from the exercise or settlement of outstanding options or restricted stock units under the Incentive Plan, and from the exercise of our warrants. In addition, sales or issuances of a substantial number of shares of our common stock, or other equity-related securities in the public markets, or the perception that such sales or issuances could occur, could depress the market price of our common stock. We do not expect, nor do our historical operating results suggest, that cash flows generated from operations will be sufficient to meet our material cash requirements in the long term. Management expects that our historical reliance on external financing, from both equity and debt financings, will continue to provide the capital necessary to meet our material cash requirements in the long term. Management has not yet determined the form such additional financing may take, but management expects that the most likely forms include one or more of the following: (i) underwritten offerings of shares of our common stock, (ii) sales of shares of our common stock under an "at the market" offering program, (iii) incurring indebtedness with one or more financial institutions, and (iv) the factoring of trade receivables. The ability of our Board of Directors to issue additional stock may prevent us from making more difficult transactions, including a sale or merger. Our Board of Directors is authorized to issue up to 10 million shares of preferred stock with powers, rights and preferences designated by it. Shares of voting or convertible preferred stock could be issued, or rights to purchase such shares could be issued, to create voting impediments or to frustrate persons seeking to effect a takeover or otherwise gain control of us. The ability of the Board of Directors to issue such additional shares of preferred stock, with rights and preferences it deems advisable, could discourage an attempt by a party to acquire control of us by tender offer or other means. Such issuances could therefore deprive stockholders of benefits that could result from such an attempt, such as the realization of a premium over the market price for their shares in a tender offer or the temporary increase in market price that such an attempt could cause. Moreover, the issuance of such additional shares of preferred stock to persons friendly to the Board of Directors could make it more difficult to remove incumbent officers and directors from office even if such change were to be favorable to stockholders generally. We have never paid and do not intend to pay cash dividends. Cash dividends have never been declared or paid on our common stock, and we do not anticipate such a declaration or payment for the foreseeable future. We expect to use future earnings, if any, to fund business growth. Therefore, stockholders will not receive any funds absent a sale of their shares of common stock. If we do not pay dividends, our common stock may be less valuable because a return on investment will only occur if our stock price appreciates The market price of our common stock has been, and may continue to be, highly volatile. During the period from our initial listing on Nasdaq on August 9, 2016 through December 31, 2022, the closing price of our common stock fluctuated from a high of $93.15 per share to a low of $1.04 per share (on a split-adjusted basis), and our stock price continues to fluctuate. The market price of our common stock may continue to fluctuate significantly in response to numerous factors, some of which are beyond our control, such as our ability to grow our revenue and customer base; the announcement of new products or product enhancements by us or our competitors; developments concerning regulatory oversight and approvals; variations in our and our competitors’ results of operations; changes in earnings estimates or recommendations by securities analysts, if our common stock is covered by analysts; successes or challenges in our collaborative arrangements or alternative funding sources; developments in the rehabilitation and industrial robotics markets; the results of product liability or intellectual property lawsuits; future issuances of common stock or other securities; the addition or departure of key personnel; announcements by us or our competitors of acquisitions, investments or strategic alliances; and general market conditions and other factors, including factors unrelated to our operating performance or otherwise disclosed herein. Table of Contents 36 Trading of our common stock is limited, which may affect our stock price. Trading of our common stock is currently conducted on Nasdaq. The liquidity of our common stock is limited, not only in terms of the number of shares that can be bought and sold at a given price, but also as it may be adversely affected by delays in the timing of transactions and low coverage by research analysts and the media, if at all. These factors may result in different prices for our common stock than might otherwise be obtained in a more liquid market and could also result in a larger spread between the bid and asked prices for our common stock. In addition, without a large public float, our common stock is less liquid than the stock of companies with broader public ownership, and, as a result, the trading prices of our common stock may be more volatile. In the absence of an active public trading market, an investor may be unable to liquidate his or her investment in our common stock. Trading of a relatively small volume of our common stock may have a greater impact on the trading price of our stock than would be the case if our public float were larger. Additionally, sales by stockholders of substantial amounts of our shares of common stock, the issuance of new shares of common stock by us or the perception that these sales may occur in the future could materially and adversely affect the market price of our common stock, and you may lose all or a portion of your investment in our common stock. Item 1B. UNRESOLVED STAFF COMMENTS None. Item 2. PROPERTIES Our principal executive offices are currently located at 101 Glacier Point, Suite A, San Rafael, California, 94901, where we lease approximately 17,000 square feet. The San Rafael office serves as headquarters for our medical device and industrial device sales segments. We currently lease manufacturing facilities in Macedonia, Ohio from Parker Hannifin Corporation to support the production and service of the Ekso Indego product lines. Outisde of the United States, we lease approximately 3,000 square feet of office space at Friesenweg 4, House 13, 4th floor, 22763 Hamburg, Germany for our European headquarters. We do not own any real property. Item 3. LEGAL PROCEEDINGS From time to time we are subject to legal proceedings and claims arising in the ordinary course of business. Based on our current knowledge, we believe that the amount or range of reasonably possible losses will not, either individually or in the aggregate, have a material adverse effect on our business, results of operations, or financial condition. The results of any litigation cannot be predicted with certainty, and an unfavorable resolution in any legal proceedings could materially affect our future business, results of operations, or financial condition. Regardless of the outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources, and other factors. For additional information, please refer to Note 16. Commitments and Contingencies in our notes to the consolidated financial statements. Item 4. MINE SAFETY DISCLOSURES Not applicable. Table of Contents 37 PART II Item 5. MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES Market Information and Dividend Policy Our common stock has been traded on the Nasdaq Capital Market under the symbol “EKSO” since August 9, 2016. Prior to August 9, 2016, our common stock was eligible for quotation and traded on the OTC Market. The quotation of our common stock on the OTC market began on or about January 16, 2014. The closing price of EKSO stock as of March 23, 2023 was $1.47. As of March 23, 2023, we had approximately 175 stockholders of record of our common stock. This number does not include stockholders whose shares are held in investment accounts by other entities. We believe that the actual number of stockholders is greater than the number of holders of record. We have never declared or paid cash dividends on our common stock and do not intend to pay cash dividends in the foreseeable future. Payment of future dividends, if any, will be at the discretion of our board of directors after taking into account various factors, including our financial condition, operating results, restrictions imposed by financing arrangements, if any, legal and regulatory restrictions on the payment of dividends, current and anticipated cash needs and other factors the board of directors deems relevant. Securities Authorized for Issuance Under Equity Compensation Plans See Item 12, “Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters” of this Annual Report on Form 10-K for information regarding securities authorized for issuance under equity compensation plans. Unregistered Sales of Equity Securities None. Issuer Purchases of Equity Securities None. Item 6. RESERVED Table of Contents 38 Item 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS You should read the following discussion and analysis of our financial condition and results of operations together with the consolidated financial statements and the related notes included elsewhere in this Annual Report on Form 10-K. This discussion and other parts of this Annual Report on Form 10-K contain forward-looking statements. that involve risks and uncertainties, such as statements of our plans, objectives, expectations and intentions, which are based on the beliefs of our management, as well as assumptions made by, and information currently available to, our management. Our actual results could differ materially from those discussed in or implied by these forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those discussed in the section of this Annual Report on Form 10-K titled “Risk Factors.” For a discussion related to the results of operations for 2021 compared to 2020, refer to “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in our 2021 Annual Report on Form 10-K filed with the SEC on February 24, 2022. Overview Our Business We design, develop, and market exoskeleton products that augment human strength, endurance and mobility. Our exoskeleton technology serves multiple markets and can be utilized both by able-bodied persons and by persons with physical disabilities. We have sold or leased devices that (i) enable individuals with neurological conditions affecting gait, including ABI, SCI and MS, to rehabilitate, and in some cases, to walk again, (ii) assist individuals with a broad range of upper extremity impairments, and (iii) allow industrial workers to perform difficult repetitive work for extended periods. We believe that the commercial opportunity for exoskeleton technology adoption is accelerating as a result of recent advancements in material technologies, electronic and electrical engineering, control technologies, and sensor and software development. Taken individually, many of these advancements have become ubiquitous in peoples’ everyday lives. We believe that we have learned how to integrate these existing technologies and wrap the result around a human being efficiently, elegantly and safely, supported by an industry leading intellectual property portfolio. We further believe that we can do so across a broad spectrum of applications, from persons with lower limb paralysis to able-bodied users. EksoHealth EksoHealth is our business unit focused on developing and marketing exoskeletons for medical applications. Our leading product in EksoHealth, the EksoNR, is a robotic exoskeleton used to provide physical therapy for patients with lower extremity impairment. EksoNR includes unique features designed specifically to assist physical therapists and other clinicians to teach patients to walk again after suffering a neurological impairment. Typical conditions that can be treated with the assistance of EksoNR include ABIs, such as stroke and traumatic brain injuries, as well as SCIs, MS, and others. The benefits of EksoNR rehabilitation can include earlier mobilization of patients, longer and more intense rehab sessions, and increased quality of sessions as compared to alternative therapies. EksoNR is typically used in clinical settings, most commonly at inpatient rehab facilities and stroke centers. EksoHealth expanded its product offerings to include Ekso Indego Therapy and Ekso Indego Personal with the HMC acquisition in the fourth quarter of 2022. The Ekso Indego devices are FDA-cleared lower-limb powered exoskeletons that enable task-specific, overground gait training to patients with weakness or paralysis in their lower extremities. Ekso Indego Personal, a light-weight exoskeleton for safe use in most home and community environments, and Ekso Indego Therapy, an adjustable exoskeleton for patients with spinal cord injury and stroke, address market opportunities across the continuum of care, particularly within outpatient facilities, complementary to those most typically addressed by the EksoNR, expanding Ekso’s product offering to home and community use markets. EksoWorks EksoWorks is our business unit focused on developing, marketing, and selling exoskeletons and other assistive tools for industrial applications. The target users for these devices are generally able-bodied, and as such the goal of these products is to reduce fatigue for workers. The benefits of fatigue reduction can include reduced rates of injuries, higher productivity, higher worker morale, and lower turnover. Currently, we primarily sell these products directly to companies that deploy them for use in their operations. Table of Contents 39

EVO, our wearable upper body exoskeleton, supersedes the EksoVest as our primary product designed to support the weight of a worker’s arms and tools, reducing the fatigue associated with working at or above shoulder height for extended periods. In 2022 the EksoWorks unit refocused its product offerings and go to market strategies, placing increased emphasis on EVO and its placement into large industrial settings within our identified target markets. We believe EVO has industrial applications across a broad range of market verticals, and the unit is currently targeting end markets in aerospace, automotive, manufacturing, commercial construction, and renewable energy. Prior to ceasing commercialization of the EksoZeroG support arm and related products and accessories, at the end of the second quarter of 2022, we manufactured and sold our EksoZeroG tool holder, which could mount on an aerial lift platform or scaffolding. 2022 Operational and Financial Highlights • Completed the HMC Acquisition including the Indego product line • Booked a total of 100 EksoHealth devices units in 2022 • Generated revenue of $12.9 million for the 2022 full year, an increase of 15% • Strong cash position of $20.5 million as of December 31, 2022 Economic and Industry Trends Our revenue is highly dependent on market demand for our exoskeleton products. This market demand is influenced by many factors including the level of awareness of robotic exoskeleton rehabilitation among the rehabilitation clinics with significant stroke, ABI, and SCI populations, the imperatives among construction and manufacturing companies to drive adoption of improved safety and health practices, the levels of reimbursements our customers will be able to receive, as well as conditions relating to overall economic growth and general business activity. Difficult and challenging economic conditions, including growing supply chain issues amidst an increasingly inflationary environment, could lead to increased price-based competition. In particular, the effects of such increasing price-based competition may have an especially significant impact on certain products that we offer, including the EksoNR and Ekso Indego, which have a lengthy sale and purchase order cycle because they are major capital expenditure items and generally require the approval of senior management at purchasing institutions. Furthermore, our business includes operations in the Americas, EMEA and APAC, so we are affected by demand for our products in those regions, as well as the strengthening or weakening of local currencies relative to the U.S. Dollar. The current economic environment is impacting our customers financially and operationally. Hospitals are experiencing staffing shortages and supply chain issues that affect their ability to provide patient care. Additionally, hospitals are facing significant financial pressure as supply chain constraints and inflation drive up operating costs and fiscal stimulus programs enacted during the COVID-19 pandemic wind down. As a consequence of the financial pressures and decreased profitability, some hospitals have indicated that they are lowering their capital investment plans and tightening their operational budgets. We believe that these factors could contribute to a reduced demand for our offerings, particularly in the United States, which may have a material adverse effect on our business, financial condition, results of operations, or cash flows. We believe the clinical need for our products has not diminished, as evidenced by clinical data showing the increased prevalence of strokes during the pandemic. We continue to engage with our current and prospective customers both onsite and virtually through video conferencing, virtual training events and online education demos to offer our support and showcase the value of our Ekso devices. While the impacts of the COVID-19 pandemic are starting to dissipate, it is possible a resurgence of COVID-19 could result in adverse effects on our business, financial condition, and results of operations in the future. Table of Contents 40 Results of Operations Consolidated Results of Operations: December 31, 2022 compared to the year ended December 31, 2021 (dollars in thousands): Years ended December 31, 2022 2021 Change % Change Revenue $ 12,912 $ 11,246 $ 1,666 15 % Cost of revenue 6,698 4,497 2,201 49 % Gross profit 6,214 6,749 (535) (8) % Gross profit % 48 % 60 % Operating expenses: Sales and marketing 7,157 7,305 (148) (2) % Research and development 3,626 2,549 1,077 42 % General and administrative 10,987 10,723 264 2 % Total operating expenses 21,770 20,577 1,193 6 % Loss from operations (15,556) (13,828) (1,728) 12 % Other (expense) income, net: Interest expense (156) (113) (43) 38 % Gain on revaluation of warrant liabilities 1,317 3,962 (2,645) nm(1) Gain on forgiveness of note payable — 1,099 (1,099) nm(1) Unrealized loss on foreign exchange (655) (867) 212 (24) % Other expense, net (30) (17) (13) nm(1) Total other income, net 476 4,064 (3,588) (88) % Net loss $ (15,080) $ (9,764) $ (5,316) 54 % (1) Not meaningful Revenue Revenue increased $1.7 million, or 15%, for the year ended December 31, 2022, compared to the same period of 2021. This increase was comprised of a $2.1 million increase in EksoHealth revenue, partially offset by a $0.4 million decrease in EksoWorks. The increase in EksoHealth revenue is primarily due to an increase in the volume of device sales in the Americas and EMEA regions for the EksoHealth reporting segment as a result of partially normalizing business conditions from the COVID-19 pandemic, particularly in the EMEA region. EksoWorks revenue decreased due to a lower volume of device sales as the unit temporarily rescaled its sales and marketing resources while it refocuses and refines its market approach. The Indego product line was acquired in December 2022. As such, the inputs from that product line were not meaningful to EksoHealth revenue in 2022. Gross Profit and Gross Margin Gross profit decreased $0.5 million, or 8%, for the year ended December 31, 2022, compared to the same period of 2021, due to a $2.2 million increase in the cost of revenue as compared to the same period of 2021. Gross margin declined to approximately 48% for the year ended December 31, 2022, compared to a gross margin of 60% for the same period in 2021. Gross margins declined across both reporting segments, EksoHealth and EksoWorks, with the decline primarily driven by inflationary pressures throughout the supply chain, elevated labor costs, and lower average selling prices of device sales for both segments. Based on management's assessment of revenue per device, service selling prices and associated cost of sales for products and revenue streams in the recently added Indego product line, we do not expect our gross margin to deviate meaningfully from historical results as a result of the HMC Acquisition. Table of Contents 41 Operating Expenses Sales and marketing expenses decreased $0.1 million, or 2%, for the year ended December 31, 2022, compared to the same period of 2021, as a result of lower compensation costs from the departure of our former Chief Commercial Officer in March 2022, With the HMC Acquisition, we added significant headcount related to sales and marketing activities, which we expect will result in an increase in our sales and marketing expenses for periods following the acquisition. Research and development expenses increased $1.1 million, or 42%, for the year ended December 31, 2022, compared to the same period of 2021, primarily due to an increase in product development activity for next generation products which drove an increase in compensation, outside services, and material usage expenses. With the HMC Acquisition, we added significant headcount related to research and development activities, and expanded our commercialized product portfolio and our pipeline of products currently under development. These additions, in conjunction with our ongoing development activities are expected to result in an increase in our research and development expenses for periods following the acquisition. General and administrative expenses increased $0.3 million, or 2%, for the year ended December 31, 2022, compared to the same period of 2021, primarily due to increased compensation and benefits expense, one time expenses for severance costs related to the departure of our Chief Executive Officer in January 2022, and costs related to the relocation of our corporate headquarters and manufacturing facility. These increases were partially offset by a decrease in legal and consulting expenses incurred in connection with business development activities. With the HMC Acquisition, we added headcount related to general and administrative activities, which we expect will result in an increase in its general and administrative expenses for periods following the acquisition. Other (Expense) Income, Net Gain on revaluation of warrant liabilities of $1.3 million for the year ended December 31, 2022, was associated with the revaluation of warrants issued in 2019, 2020 and 2021. Gain on revaluation of warrant liabilities of $4.0 million for the year ended December 31, 2021, was related to warrants issued in 2019, 2020 and 2021. Gains and losses on revaluation of warrants are primarily driven by changes in our stock price. Gain on forgiveness of note payable of $1.1 million was recorded for the year ended December 31, 2021, as a result of the PPP loan forgiveness approval we received from our lender and the U.S. Small Business Administration in June 2021. There was no comparable amount for the same period of 2022. Unrealized loss on foreign exchange was $0.7 million for the year ended December 31, 2022, compared to unrealized loss on foreign exchange of $0.9 million for the same period of 2021, mostly due to foreign currency exchange rate fluctuations producing unrealized gains and losses on our inter-company monetary assets and liabilities. Liquidity and Capital Resources Since our inception, we have devoted substantially all of our efforts toward the development of exoskeletons for the medical and industrial markets, toward the commercialization of medical exoskeletons to rehabilitation centers and toward raising capital. We have financed our operations primarily through the issuance and sale of equity securities for cash consideration and through bank debt. As of December 31, 2022, we had working capital of $21.8 million, compared to working capital of $40.9 million as of December 31, 2021. The decrease in working capital is primarily due to cash outflows from operations of $14.7 million and cash outflows from investing activities of $5.2 million mostly related to the HMC Acquisition. Our cash as of December 31, 2022 consisted of bank deposits with third party financial institutions. As of December 31, 2022, of our $20.5 million of cash, $19.6 million was held domestically and $0.9 million was held by our foreign subsidiaries. As of December 31, 2022, we had an accumulated deficit of $223.9 million and cash on hand of $20.5 million. Largely as a result of significant research and development activities related to our advanced technology and commercialization of such technology into our medical device business, we have incurred significant operating losses and negative cash flows from operations since inception. We have incurred net losses of $15.1 million and $9.8 million for the years ended December 31, 2022 and 2021, respectively. In the year ended December 31, 2022, we used $14.7 million of cash in our operations. In October 2020, we entered into an At The Market Offering Agreement (the "ATM Agreement") with Wainwright, under which we may issue and sell shares of our common stock from time to time, to or through Wainwright. Offers and sales of Table of Contents 42 shares of common stock by us through Wainwright may be made by any method deemed to be an “at the market offering” as defined under SEC Rule 415 or in privately negotiated transactions, subject to certain conditions. Such shares may be offered pursuant to the registration statement on Form S-3 (File No. 333-239203) (the “Registration Statement”), which was declared effective by the SEC on June 26, 2020, and a related prospectus supplement filed with the SEC on October 9, 2020 (the “ATM Prospectus”). Pursuant to the Registration Statement and the ATM Prospectus, shares having an aggregate offering price of up to $7.5 million may be offered and sold, subject to certain SEC rules limiting the amount of shares of our common stock that we may sell under the Registration Statement. Under the ATM Agreement, shares of our common stock may not be sold for a price lower than $6.75 per share. During the year ended December 31, 2022, we did not sell any shares under the ATM Agreement. As of December 31, 2022, we had $6.7 million available for future offerings under the prospectus filed with respect to the ATM Agreement. In February 2021, the Company entered into an amended and restated underwriting agreement (the "Underwriting Agreement") with H.C. Wainwright & Co., LLC ("Wainwright"), to sell 3,902 shares of the Company's common stock for a public price of $10.25 per share, for gross proceeds of $40.0 million (the "February 2021 Offering"). The Company received net proceeds of $36.5 million from the February 2021 Offering. In addition to the cash proceeds received in the February 2021 Offering, the Company received $1.4 million in cash proceeds from the exercise of warrants issued in connection with financings in June 2020 and May 2019 and $0.8 million in net proceeds of $0.8 million from our “at the market offering” program. As described in Note 10. Notes Payable, Net in the notes to our consolidated financial statements, borrowings under our secured term loan agreement with Pacific Western Bank have a requirement of minimum cash on hand equivalent to the current outstanding principal balance, which is due in full in August 2023. As of December 31, 2022, $2.0 million of cash must remain as restricted. After considering cash restrictions, effective unrestricted cash as of December 31, 2022 is estimated to be $18.5 million. With this unrestricted cash balance, we believe that we currently have sufficient cash to fund our operations twelve months beyond the issuance of the financials statements. Cash and Restricted Cash The following table summarizes the sources and uses of cash for the periods stated (in thousands): Years ended December 31, 2022 2021 Cash and restricted cash, beginning of year $ 40,406 $ 12,862 Net cash used in operating activities (14,688) (11,156) Net cash used in investing activities (5,175) (59) Net cash provided by financing activities — 38,712 Effect of exchange rate changes on cash (18) 47 Cash and restricted cash, end of year $ 20,525 $ 40,406 Net Cash Used in Operating Activities Net cash used in operating activities increased $3.5 million for the year ended December 31, 2022, compared to the same period of 2021, primarily due to payment of business development expenses incurred in late 2021, employee compensation related to higher headcount, increased inventory purchases, and costs related to moving our new headquarters and manufacturing facility to San Rafael, California. Net Cash Used in Investing Activities Net cash used in investing activities increased $5.1 million for the year ended December 31, 2022, compared to the same period of 2021 due to payment of $5.0 million for the HMC Acquisition and leasehold improvements for our new headquarters and manufacturing facility in San Rafael, California. Net Cash Provided by Financing Activities Net cash provided by financing activities of $38.7 million for the year ended December 31, 2021, was generated from the sale of common stock and warrants for net proceeds of $36.5 million in connection with the equity financing, net proceeds of $0.8 million from our “at the market offering” program, and proceeds of $1.4 million from the exercise of warrants. There were no comparable amounts of cash inflows generated in financing activities for the year ended December 31, 2022. Table of Contents 43

Material Cash Requirements The Company's material cash requirements include the following items, some of which are represented in the table of Contractual Obligations and Commitments: (1) employee wages, benefits and incentives, (2) the procurement of raw materials and components to support the manufacturing and sale of the Company's products, (3) expenditures for the ongoing improvement and development of existing and new technologies, (4) debt repayments (for additional information see Note 10 in the notes to the Company's consolidated financial statements included elsewhere in the Annual Report on Form 10-K), and (5) operating lease payments (for additional information see Note 11. Lease Obligations in the notes to our consolidated financial statements included elsewhere in the Annual Report on Form 10-K). The Company expects that its operating cash requirements in the near term will continue to exceed cash provided by operations with the additional headcount and product development activities assumed in the HMC Acquisition. Additionally, the Company's term loan with Pacific Western Bank will mature in August 2023 requiring cash outflows of $2.0 million for the repayment of principal. Principal payments on the Company's promissory note with Parker Hannifin begin in December 2023. Notwithstanding the Company's ability to refinance its maturing loan obligation, coordinate with its suppliers to delay the receipt of materials on order, or delay product development activities, management believes it has sufficient cash on hand of $20.5 million at December 31, 2022 to meet its cash requirements twelve months from the issuance of the financial statements. The Company does not expect, nor do our historical operating results suggest, that cash flows generated from operations will be sufficient to meet our material cash requirements in the long term. Management expects that the Company's historical reliance on external financing, from both equity and debt financings, will continue to provide the capital necessary to meet its material cash requirements in the long term. Management has not yet determined the form such additional financing may take, but management expects that the most likely forms include one or more of the following: (i) underwritten offerings of shares of our common stock (ii) sales of shares of our common stock under an "at the market" offering program, (iii) incurring indebtedness with one or more financial institutions, and (iv) the factoring of trade receivables. Contractual Obligations and Commitments The following table summarizes our outstanding contractual obligations, including interest payments, as of December 31, 2022 and the effect those obligations are expected to have on our liquidity and cash flows in future periods (in thousands): Payments Due By Period Total Less than one year 1-3 Years 3-5 Years After 5 Years Term loan $ 2,107 $ 2,107 $ — $ — $ — Promissory Note 5,000 313 2,500 2,187 Facility operating leases 1,578 408 821 349 — Purchase obligations 3,480 3,480 — — — Total $ 12,165 $ 6,308 $ 3,321 $ 2,536 $ — In response to, or in anticipation of, supplier disruptions and extended lead times, in 2022, we stockpiled certain components or raw materials to help prevent disruption in our production of the EksoNR and Ekso Indego Therapy and Ekso Indego Personal devices. Such purchasing behavior is a contributing factor to the increase in purchase obligations as compared to prior periods. These actions have, and could continue to have, a short-term adverse impact on our cash used in operating activities and increase our inventory balance. Obligations related to these activities are reflected in the line purchase obligations in the table above. Refer to Note 16. Commitments and Contingencies in our notes to the consolidated financial statements for additional information regarding our contractual obligations and commitments. Off-Balance Sheet Arrangements As of December 31, 2022, we did not have any significant off-balance sheet arrangements, as defined in Item 303(a)(4)(ii) of SEC Regulation S-K promulgated under the Exchange Act. Table of Contents 44 Critical Accounting Policies and Estimates Our discussion and analysis of our financial condition and results of operations is based upon our consolidated financial statements, which have been prepared in accordance with U.S. GAAP. The preparation of these consolidated financial statements requires us to make estimates, judgments and assumptions that affect the reported amounts of assets, liabilities, revenue and expenses, and the related disclosure of contingent assets and liabilities. We base our estimates on historical experience and on various other assumptions that we believe are reasonable under the circumstances. Our estimates form the basis for our judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates. Our most critical accounting estimates include: • the standalone selling prices used to allocate the contract consideration to the individual performance obligations in our device sales arrangements, which impacts revenue recognition; • the unobservable inputs and assumptions used by management in estimating the fair value of our warrant liabilities, which impacts net income or loss; • the valuation of inventory, which impacts gross profit margins; and • the estimates made regarding the recoverability of our net deferred tax asset, which impacts our financial condition. Standalone Selling Prices Our device sales arrangements contain multiple products and services, most often including the device(s) and service, both of which we have identified as distinct performance obligations. Revenue is allocated to each performance obligation based on its relative standalone selling price. Standalone selling prices are based on observable prices at which we separately sell the products or services. If a standalone selling price is not directly observable, then we estimate the standalone selling prices considering market conditions and entity-specific factors including, but not limited to, features and functionality of the products and services, geographies, type of customer, and gross margin targets. Changes in the relative standalone selling price between devices and service can have an impact on how transaction prices are allocated between revenue and deferred revenue. Warrant Liabilities We use the Black-Scholes option-pricing model to value our warrant liabilities at each reporting period, which requires the input of highly subjective assumptions, most notably the estimated volatility of our common stock over the expected term. We use our historical common stock volatility to estimate expected volatility over the warrant terms. Management must also make uncertain estimates regarding the likelihood and timing of certain future events for application of the Lattice Model for the valuation of certain warrants. Changes in these assumptions could have potential material impacts on the estimated fair value of warrant liabilities. During the year ended December 31, 2022, management made changes to its estimates regarding the likelihood and timing of future events. We do not believe the revision resulted in a material impact to the estimated fair value of warrant liabilities measured using the Lattice Model. Inventory Valuation Inventory is stated at the lower of cost or net realizable value. Cost is computed using the standard cost method which approximates actual cost on a first-in, first-out basis. The cost basis of our inventory is reduced for any products that are considered excessive or obsolete based upon assumptions about future demand and market conditions. If actual future demand or market conditions are less favorable than those projected by management, additional inventory write-downs may be required, which could have a material adverse effect on the results of our operations. Deferred Tax Asset We estimate a valuation allowance in consideration of the realizability of our net deferred tax assets, primarily based on our assessment of the timing, likelihood and amounts of potential future income during which such items become deductible. It is inherently difficult and subjective to estimate such amounts, as we must determine the probability of various possible outcomes and estimate future amounts. Management does not believe it is more likely than not that we will generate future income in a time frame and amount sufficient to realize our net deferred tax assets. Changes in management's estimate of future income in the timeframe during which the temporary differences and carryforwards comprising our deferred tax assets become deductible could result in a material impact to our financial position including the recognition of a net deferred tax asset. Accounting Policies Table of Contents 45 An accounting policy is considered to be critical if it requires an accounting estimate to be made based on assumptions about matters that are highly uncertain at the time the estimate is made, and if different estimates that reasonably could have been used, or changes in the accounting estimate that are reasonably likely to occur, could materially impact the consolidated financial statements. We believe that our critical accounting policies reflect the more significant estimates and assumptions used in the preparation of the consolidated financial statements. Refer to Note. 2 Summary of Significant Accounting Policies and Estimates in the notes to our consolidated financial statements included elsewhere in this Annual Report on Form 10-K. Recent Accounting Pronouncements See Note 2. Summary of Significant Accounting Policies and Estimates—Recent Accounting Pronouncements in the notes to our consolidated financial statements for a discussion of new accounting pronouncements. Table of Contents 46 ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK Foreign Currency Risk We report our financial results in U.S. dollars; however, we conduct business in foreign countries. For U.S. reporting purposes, we translate all assets and liabilities of our non-U.S. subsidiaries at the period-end exchange rate, equity at historical exchange rates, and revenue and expenses at the average exchange rates in effect during the periods. The net effect of these translation adjustments is shown in the accompanying consolidated financial statements as a component of stockholders’ equity. We generate a portion of our revenue and collect receivables in foreign currencies outside of the U.S. and, as such, we have foreign currency exposure. Currently, we sell our products mainly in United States dollars, Euros, and Singapore dollars although we may in the future transact business in other currencies. Future fluctuations in the foreign exchange rates of these currencies can result in foreign exchange gains and losses which may impact our financial results. In the past, we have not hedged our exposures to foreign currencies or entered into any other derivative instruments and we have no current plans to do so. For the year ended December 31, 2022, sales denominated in foreign currencies were approximately 44% of total revenue. A hypothetical 10% increase in the United States dollar exchange rate used would have resulted in a $0.6 million decrease to revenues for 2022. Interest Rate Risk Our exposure to market rate risk for changes in interest rates relates primarily to our term loan. The variable interest rate related to our long-term debt is charged at the greater of 0.50% above the variable rate of interest announced by the lender as its “prime rate” then in effect or 4.50%. A hypothetical 10% change in the lender's prime rate would have an immaterial impact on our annualized interest expense. Table of Contents 47

ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA Table of Contents The following consolidated financial statements are filed as part of this Annual Report on Form 10-K Page Number Report of Independent Registered Public Accounting Firm 49 Consolidated Balance Sheets as of December 31, 2022 and 2021 51 Consolidated Statements of Operations and Comprehensive Loss for the years ended December 31, 2022 and 2021 52 Consolidated Statements of Stockholders’ Equity for the years ended December 31, 2022 and 2021 53 Consolidated Statements of Cash Flows for the years ended December 31, 2022 and 2021 54 Notes to Consolidated Financial Statements 56 Table of Contents 48 REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM To the Stockholders and Board of Directors Ekso Bionics Holdings, Inc. Opinion on the Consolidated Financial Statements We have audited the accompanying consolidated balance sheets of Ekso Bionics Holdings, Inc. (the “Company”) as of December 31, 2022 and 2021, the related consolidated statements of operations and comprehensive loss, stockholders’ equity, and cash flows for each of the two years in the period ended December 31, 2022, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2022 and 2021, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2022, in conformity with accounting principles generally accepted in the United States of America. Basis for Opinion These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB. We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion. Critical Audit Matter The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matter does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing separate opinions on the critical audit matter or on the accounts or disclosures to which they relate. Revenue Recognition – transaction price allocation for contracts with customers containing multiple performance obligations Description of the Matter As described in Note 2 to the consolidated financial statements, the Company’s contracts with customers may contain multiple performance obligations, which are accounted for separately if they are distinct. In such cases, the transaction price is then allocated to the distinct performance obligations on a relative standalone selling price basis and revenue is recognized when the distinct performance obligation is satisfied. For example, device revenue is recognized at the point in time that the customer takes control of the device, generally upon shipment, and subscription and service revenues are recognized over time as the services are performed. Auditing the Company’s revenue recognition was challenging, specifically related to the identification and determination of the distinct performance obligations, the allocation of the transaction price to the identified performance obligations and the timing of revenue recognition. For example, certain arrangements required judgment to determine the distinct performance obligations, Table of Contents 49 how the transaction price is allocated to the identified performance obligations, and the appropriate timing of revenue recognition. How We Addressed the Matter in Our Audit We obtained an understanding and evaluated the design of the Company’s process and controls to determine the distinct performance obligations, allocation of the transaction price to the identified performance obligations and the timing of revenue recognition. Among the procedures we performed to test the determination of the distinct performance obligations, allocations of the transaction price to the identified performance obligations and the timing of revenue recognition, we read executed contracts and purchase orders to understand the rights and obligations conveyed in the contractual arrangement, evaluated management’s assessment of the performance obligations and whether they were distinct, determined the reasonableness of the standalone selling price used by management in the allocation of the transaction price to the performance obligations, and tested the timing of revenue recognition for a sample of individual sales transactions. We evaluated the accuracy of the Company’s accounting conclusions, specifically related to the identification and determination of distinct performance obligations, allocation of the transaction price to the identified performance obligations, and the timing of revenue recognition. /s/ WithumSmith+Brown, PC We have served as the Company's auditor since 2010. San Francisco, California March 28, 2023 PCAOB ID Number 100 Table of Contents 50 Ekso Bionics Holdings, Inc. Consolidated Balance Sheets (In thousands, except par value amounts) December 31, 2022 2021 Assets Current assets: Cash and restricted cash $ 20,525 $ 40,406 Accounts receivable, net of allowances of $40 and $28, respectively 4,625 4,662 Inventories 5,187 2,242 Prepaid expenses and other current assets 700 485 Total current assets 31,037 47,795 Property and equipment, net 2,680 991 Right-of-use assets 1,307 216 Intangible assets, net 5,217 — Goodwill 431 — Other assets 231 164 Total assets $ 40,903 $ 49,166 Liabilities and Stockholders' Equity Current liabilities: Accounts payable $ 3,151 $ 3,107 Accrued liabilities 2,278 2,299 Deferred revenues, current 1,121 1,220 Notes payable, current 2,310 — Lease liabilities, current 341 229 Total current liabilities 9,201 6,855 Deferred revenues 1,032 1,475 Notes payable, net 3,767 1,993 Lease liabilities 1,087 — Warrant liabilities 233 1,550 Other non-current liabilities 141 74 Total liabilities 15,461 11,947 Commitments and contingencies (Note 16) Stockholders' equity: Convertible preferred stock, $0.001 par value; 10,000 shares authorized; no shares issued and outstanding at December 31, 2022 and 2021 — — Common stock, $0.001 par value; 141,429 shares authorized; 13,203 and 12,693 shares issued and outstanding at December 31, 2022 and 2021, respectively 13 13 Additional paid-in capital 248,813 246,090 Accumulated other comprehensive gain (loss) 563 (17) Accumulated deficit (223,947) (208,867) Total stockholders' equity 25,442 37,219 Total liabilities and stockholders' equity $ 40,903 $ 49,166 See accompanying notes to consolidated financial statements Table of Contents 51
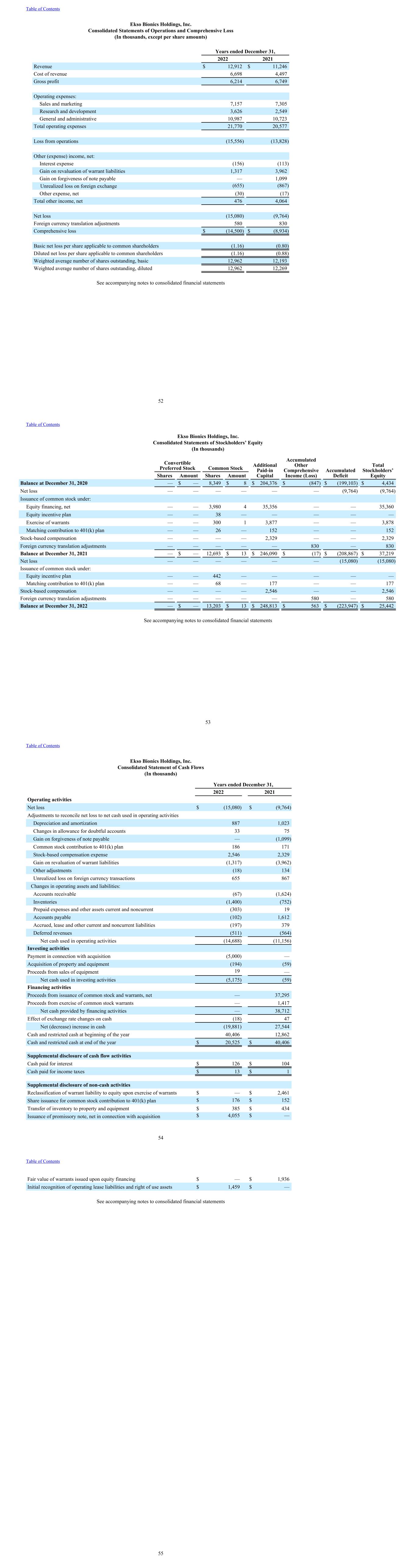
Ekso Bionics Holdings, Inc. Consolidated Statements of Operations and Comprehensive Loss (In thousands, except per share amounts) Years ended December 31, 2022 2021 Revenue $ 12,912 $ 11,246 Cost of revenue 6,698 4,497 Gross profit 6,214 6,749 Operating expenses: Sales and marketing 7,157 7,305 Research and development 3,626 2,549 General and administrative 10,987 10,723 Total operating expenses 21,770 20,577 Loss from operations (15,556) (13,828) Other (expense) income, net: Interest expense (156) (113) Gain on revaluation of warrant liabilities 1,317 3,962 Gain on forgiveness of note payable — 1,099 Unrealized loss on foreign exchange (655) (867) Other expense, net (30) (17) Total other income, net 476 4,064 Net loss (15,080) (9,764) Foreign currency translation adjustments 580 830 Comprehensive loss $ (14,500) $ (8,934) Basic net loss per share applicable to common shareholders (1.16) (0.80) Diluted net loss per share applicable to common shareholders (1.16) (0.88) Weighted average number of shares outstanding, basic 12,962 12,193 Weighted average number of shares outstanding, diluted 12,962 12,269 See accompanying notes to consolidated financial statements Table of Contents 52 Ekso Bionics Holdings, Inc. Consolidated Statements of Stockholders’ Equity (In thousands) Balance at December 31, 2020 — $ — 8,349 $ 8 $ 204,376 $ (847) $ (199,103) $ 4,434 Net loss — — — — — — (9,764) (9,764) Issuance of common stock under: Equity financing, net — — 3,980 4 35,356 — — 35,360 Equity incentive plan — — 38 — — — — — Exercise of warrants — — 300 1 3,877 — — 3,878 Matching contribution to 401(k) plan — — 26 — 152 — — 152 Stock-based compensation — — — — 2,329 — — 2,329 Foreign currency translation adjustments — — — — — 830 — 830 Balance at December 31, 2021 — $ — 12,693 $ 13 $ 246,090 $ (17) $ (208,867) $ 37,219 Net loss — — — — — — (15,080) (15,080) Issuance of common stock under: Equity incentive plan — — 442 — — — — — Matching contribution to 401(k) plan — — 68 — 177 — — 177 Stock-based compensation — — — — 2,546 — — 2,546 Foreign currency translation adjustments — — — — — 580 — 580 Balance at December 31, 2022 — $ — 13,203 $ 13 $ 248,813 $ 563 $ (223,947) $ 25,442 Convertible Preferred Stock Common Stock Additional Paid-in Capital Accumulated Other Comprehensive Income (Loss) Accumulated Deficit Total Stockholders’ EquityShares Amount Shares Amount See accompanying notes to consolidated financial statements Table of Contents 53 Ekso Bionics Holdings, Inc. Consolidated Statement of Cash Flows (In thousands) Years ended December 31, 2022 2021 Operating activities Net loss $ (15,080) $ (9,764) Adjustments to reconcile net loss to net cash used in operating activities Depreciation and amortization 887 1,023 Changes in allowance for doubtful accounts 33 75 Gain on forgiveness of note payable — (1,099) Common stock contribution to 401(k) plan 186 171 Stock-based compensation expense 2,546 2,329 Gain on revaluation of warrant liabilities (1,317) (3,962) Other adjustments (18) 134 Unrealized loss on foreign currency transactions 655 867 Changes in operating assets and liabilities: Accounts receivable (67) (1,624) Inventories (1,400) (752) Prepaid expenses and other assets current and noncurrent (303) 19 Accounts payable (102) 1,612 Accrued, lease and other current and noncurrent liabilities (197) 379 Deferred revenues (511) (564) Net cash used in operating activities (14,688) (11,156) Investing activities Payment in connection with acquisition (5,000) — Acquisition of property and equipment (194) (59) Proceeds from sales of equipment 19 — Net cash used in investing activities (5,175) (59) Financing activities Proceeds from issuance of common stock and warrants, net — 37,295 Proceeds from exercise of common stock warrants — 1,417 Net cash provided by financing activities — 38,712 Effect of exchange rate changes on cash (18) 47 Net (decrease) increase in cash (19,881) 27,544 Cash and restricted cash at beginning of the year 40,406 12,862 Cash and restricted cash at end of the year $ 20,525 $ 40,406 Supplemental disclosure of cash flow activities Cash paid for interest $ 126 $ 104 Cash paid for income taxes $ 13 $ 1 Supplemental disclosure of non-cash activities Reclassification of warrant liability to equity upon exercise of warrants $ — $ 2,461 Share issuance for common stock contribution to 401(k) plan $ 176 $ 152 Transfer of inventory to property and equipment $ 385 $ 434 Issuance of promissory note, net in connection with acquisition $ 4,055 $ — Table of Contents 54 Fair value of warrants issued upon equity financing $ — $ 1,936 Initial recognition of operating lease liabilities and right of use assets $ 1,459 $ — See accompanying notes to consolidated financial statements Table of Contents 55
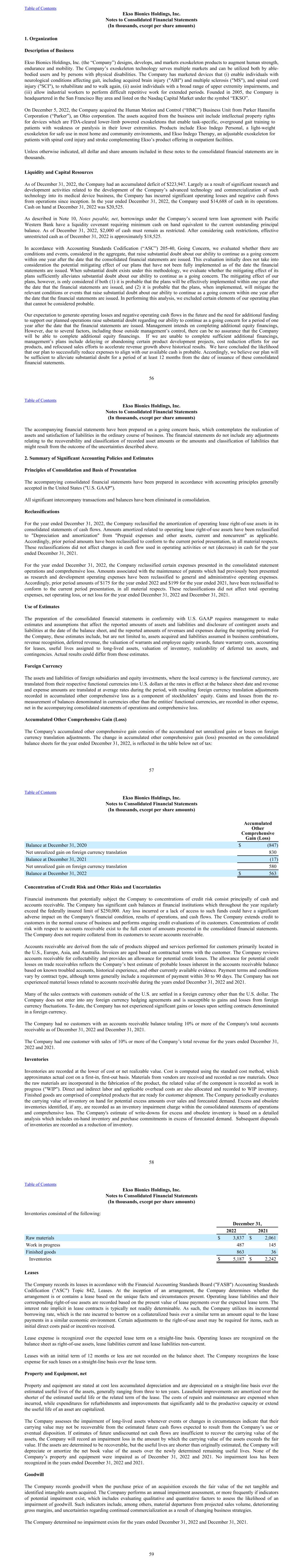
1. Organization Description of Business Ekso Bionics Holdings, Inc. (the “Company”) designs, develops, and markets exoskeleton products to augment human strength, endurance and mobility. The Company’s exoskeleton technology serves multiple markets and can be utilized both by able- bodied users and by persons with physical disabilities. The Company has marketed devices that (i) enable individuals with neurological conditions affecting gait, including acquired brain injury ("ABI") and multiple sclerosis ("MS"), and spinal cord injury ("SCI"), to rehabilitate and to walk again, (ii) assist individuals with a broad range of upper extremity impairments, and (iii) allow industrial workers to perform difficult repetitive work for extended periods. Founded in 2005, the Company is headquartered in the San Francisco Bay area and listed on the Nasdaq Capital Market under the symbol “EKSO”. On December 5, 2022, the Company acquired the Human Motion and Control (“HMC”) Business Unit from Parker Hannifin Corporation (“Parker”), an Ohio corporation. The assets acquired from the business unit include intellectual property rights for devices which are FDA-cleared lower-limb powered exoskeletons that enable task-specific, overground gait training to patients with weakness or paralysis in their lower extremities. Products include Ekso Indego Personal, a light-weight exoskeleton for safe use in most home and community environments, and Ekso Indego Therapy, an adjustable exoskeleton for patients with spinal cord injury and stroke complementing Ekso’s product offering in outpatient facilities. Unless otherwise indicated, all dollar and share amounts included in these notes to the consolidated financial statements are in thousands. Liquidity and Capital Resources As of December 31, 2022, the Company had an accumulated deficit of $223,947. Largely as a result of significant research and development activities related to the development of the Company’s advanced technology and commercialization of such technology into its medical device business, the Company has incurred significant operating losses and negative cash flows from operations since inception. In the year ended December 31, 2022, the Company used $14,688 of cash in its operations. Cash on hand at December 31, 2022 was $20,525. As described in Note 10, Notes payable, net, borrowings under the Company’s secured term loan agreement with Pacific Western Bank have a liquidity covenant requiring minimum cash on hand equivalent to the current outstanding principal balance. As of December 31, 2022, $2,000 of cash must remain as restricted. After considering cash restrictions, effective unrestricted cash as of December 31, 2022 is approximately $18,525. In accordance with Accounting Standards Codification (“ASC”) 205-40, Going Concern, we evaluated whether there are conditions and events, considered in the aggregate, that raise substantial doubt about our ability to continue as a going concern within one year after the date that the consolidated financial statements are issued. This evaluation initially does not take into consideration the potential mitigating effect of our plans that have not been fully implemented as of the date the financial statements are issued. When substantial doubt exists under this methodology, we evaluate whether the mitigating effect of its plans sufficiently alleviates substantial doubt about our ability to continue as a going concern. The mitigating effect of our plans, however, is only considered if both (1) it is probable that the plans will be effectively implemented within one year after the date that the financial statements are issued, and (2) it is probable that the plans, when implemented, will mitigate the relevant conditions or events that raise substantial doubt about our ability to continue as a going concern within one year after the date that the financial statements are issued. In performing this analysis, we excluded certain elements of our operating plan that cannot be considered probable. Our expectation to generate operating losses and negative operating cash flows in the future and the need for additional funding to support our planned operations raise substantial doubt regarding our ability to continue as a going concern for a period of one year after the date that the financial statements are issued. Management intends on completing additional equity financings, However, due to several factors, including those outside management’s control, there can be no assurance that the Company will be able to complete additional equity financings. If we are unable to complete sufficient additional financings, management’s plans include delaying or abandoning certain product development projects, cost reduction efforts for our products, and refocused sales efforts to accelerate revenue growth above historical results. We have concluded the likelihood that our plan to successfully reduce expenses to align with our available cash is probable. Accordingly, we believe our plan will be sufficient to alleviate substantial doubt for a period of at least 12 months from the date of issuance of these consolidated financial statements. Table of Contents Ekso Bionics Holdings, Inc. Notes to Consolidated Financial Statements (In thousands, except per share amounts) 56 The accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and satisfaction of liabilities in the ordinary course of business. The financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might result from the outcome of the uncertainties described above. 2. Summary of Significant Accounting Policies and Estimates Principles of Consolidation and Basis of Presentation The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States ("U.S. GAAP"). All significant intercompany transactions and balances have been eliminated in consolidation. Reclassifications For the year ended December 31, 2022, the Company reclassified the amortization of operating lease right-of-use assets in its consolidated statements of cash flows. Amounts amortized related to operating lease right-of-use assets have been reclassified to "Depreciation and amortization" from "Prepaid expenses and other assets, current and noncurrent" as applicable. Accordingly, prior period amounts have been reclassified to conform to the current period presentation, in all material respects. These reclassifications did not affect changes in cash flow used in operating activities or net (decrease) in cash for the year ended December 31, 2021. For the year ended December 31, 2022, the Company reclassified certain expenses presented in the consolidated statement operations and comprehensive loss. Amounts associated with the maintenance of patents which had previously been presented as research and development operating expenses have been reclassified to general and administrative operating expenses. Accordingly, prior period amounts of $175 for the year ended 2022 and $199 for the year ended 2021, have been reclassified to conform to the current period presentation, in all material respects. These reclassifications did not affect total operating expenses, net operating loss, or net loss for the year ended December 31, 2022 and December 31, 2021. Use of Estimates The preparation of the consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the balance sheet, and the reported amounts of revenues and expenses during the reporting period. For the Company, these estimates include, but are not limited to, assets acquired and liabilities assumed in business combinations, revenue recognition, deferred revenue, the valuation of warrants and employee equity awards, future warranty costs, accounting for leases, useful lives assigned to long-lived assets, valuation of inventory, realizability of deferred tax assets, and contingencies. Actual results could differ from those estimates. Foreign Currency The assets and liabilities of foreign subsidiaries and equity investments, where the local currency is the functional currency, are translated from their respective functional currencies into U.S. dollars at the rates in effect at the balance sheet date and revenue and expense amounts are translated at average rates during the period, with resulting foreign currency translation adjustments recorded in accumulated other comprehensive loss as a component of stockholders’ equity. Gains and losses from the re- measurement of balances denominated in currencies other than the entities' functional currencies, are recorded in other expense, net in the accompanying consolidated statements of operations and comprehensive loss. Accumulated Other Comprehensive Gain (Loss) The Company's accumulated other comprehensive gain consists of the accumulated net unrealized gains or losses on foreign currency translation adjustments. The change in accumulated other comprehensive gain (loss) presented on the consolidated balance sheets for the year ended December 31, 2022, is reflected in the table below net of tax: Table of Contents Ekso Bionics Holdings, Inc. Notes to Consolidated Financial Statements (In thousands, except per share amounts) 57 Accumulated Other Comprehensive Gain (Loss) Balance at December 31, 2020 $ (847) Net unrealized gain on foreign currency translation 830 Balance at December 31, 2021 (17) Net unrealized gain on foreign currency translation 580 Balance at December 31, 2022 $ 563 Concentration of Credit Risk and Other Risks and Uncertainties Financial instruments that potentially subject the Company to concentrations of credit risk consist principally of cash and accounts receivable. The Company has significant cash balances at financial institutions which throughout the year regularly exceed the federally insured limit of $250,000. Any loss incurred or a lack of access to such funds could have a significant adverse impact on the Company's financial condition, results of operations, and cash flows. The Company extends credit to customers in the normal course of business and performs ongoing credit evaluations of its customers. Concentrations of credit risk with respect to accounts receivable exist to the full extent of amounts presented in the consolidated financial statements. The Company does not require collateral from its customers to secure accounts receivable. Accounts receivable are derived from the sale of products shipped and services performed for customers primarily located in the U.S., Europe, Asia, and Australia. Invoices are aged based on contractual terms with the customer. The Company reviews accounts receivable for collectability and provides an allowance for potential credit losses. The allowance for potential credit losses on trade receivables reflects the Company’s best estimate of probable losses inherent in the accounts receivable balance based on known troubled accounts, historical experience, and other currently available evidence. Payment terms and conditions vary by contract type, although terms generally include a requirement of payment within 30 to 90 days. The Company has not experienced material losses related to accounts receivable during the years ended December 31, 2022 and 2021. Many of the sales contracts with customers outside of the U.S. are settled in a foreign currency other than the U.S. dollar. The Company does not enter into any foreign currency hedging agreements and is susceptible to gains and losses from foreign currency fluctuations. To date, the Company has not experienced significant gains or losses upon settling contracts denominated in a foreign currency. The Company had no customers with an accounts receivable balance totaling 10% or more of the Company's total accounts receivable as of December 31, 2022 and December 31, 2021. The Company had one customer with sales of 10% or more of the Company’s total revenue for the years ended December 31, 2022 and 2021. Inventories Inventories are recorded at the lower of cost or net realizable value. Cost is computed using the standard cost method, which approximates actual cost on a first-in, first-out basis. Materials from vendors are received and recorded as raw materials. Once the raw materials are incorporated in the fabrication of the product, the related value of the component is recorded as work in progress ("WIP"). Direct and indirect labor and applicable overhead costs are also allocated and recorded to WIP inventory. Finished goods are comprised of completed products that are ready for customer shipment. The Company periodically evaluates the carrying value of inventory on hand for potential excess amounts over sales and forecasted demand. Excess and obsolete inventories identified, if any, are recorded as an inventory impairment charge within the consolidated statements of operations and comprehensive loss. The Company's estimate of write-downs for excess and obsolete inventory is based on a detailed analysis which includes on-hand inventory and purchase commitments in excess of forecasted demand. Subsequent disposals of inventories are recorded as a reduction of inventory. Table of Contents Ekso Bionics Holdings, Inc. Notes to Consolidated Financial Statements (In thousands, except per share amounts) 58 Inventories consisted of the following: December 31, 2022 2021 Raw materials $ 3,837 $ 2,061 Work in progress 487 145 Finished goods 863 36 Inventories $ 5,187 $ 2,242 Leases The Company records its leases in accordance with the Financial Accounting Standards Board ("FASB") Accounting Standards Codification ("ASC") Topic 842, Leases. At the inception of an arrangement, the Company determines whether the arrangement is or contains a lease based on the unique facts and circumstances present. Operating lease liabilities and their corresponding right-of-use assets are recorded based on the present value of lease payments over the expected lease term. The interest rate implicit in lease contracts is typically not readily determinable. As such, the Company utilizes its incremental borrowing rate, which is the rate incurred to borrow on a collateralized basis over a similar term an amount equal to the lease payments in a similar economic environment. Certain adjustments to the right-of-use asset may be required for items, such as initial direct costs paid or incentives received. Lease expense is recognized over the expected lease term on a straight-line basis. Operating leases are recognized on the balance sheet as right-of-use assets, lease liabilities current and lease liabilities non-current. Leases with an initial term of 12 months or less are not recorded on the balance sheet. The Company recognizes the lease expense for such leases on a straight-line basis over the lease term. Property and Equipment, net Property and equipment are stated at cost less accumulated depreciation and are depreciated on a straight-line basis over the estimated useful lives of the assets, generally ranging from three to ten years. Leasehold improvements are amortized over the shorter of the estimated useful life or the related term of the lease. The costs of repairs and maintenance are expensed when incurred, while expenditures for refurbishments and improvements that significantly add to the productive capacity or extend the useful life of an asset are capitalized. The Company assesses the impairment of long-lived assets whenever events or changes in circumstances indicate that their carrying value may not be recoverable from the estimated future cash flows expected to result from the Company’s use or eventual disposition. If estimates of future undiscounted net cash flows are insufficient to recover the carrying value of the assets, the Company will record an impairment loss in the amount by which the carrying value of the assets exceeds the fair value. If the assets are determined to be recoverable, but the useful lives are shorter than originally estimated, the Company will depreciate or amortize the net book value of the assets over the newly determined remaining useful lives. None of the Company’s property and equipment were impaired as of December 31, 2022 and 2021. No impairment loss has been recognized in the years ended December 31, 2022 and 2021. Goodwill The Company records goodwill when the purchase price of an acquisition exceeds the fair value of the net tangible and identified intangible assets acquired. The Company performs an annual impairment assessment, or more frequently if indicators of potential impairment exist, which includes evaluating qualitative and quantitative factors to assess the likelihood of an impairment of goodwill. Such indicators include, among others, material departures from projected sales volume, deteriorating gross margins, and uncertainties regarding continued commercialization as a result of changing business strategies. The Company determined no impairment exists for the years ended December 31, 2022 and December 31, 2021. Table of Contents Ekso Bionics Holdings, Inc. Notes to Consolidated Financial Statements (In thousands, except per share amounts) 59

Intangible Assets Other intangible assets include developed technology, acquired intellectual property, and customer relationships, in the case of finite-lived intangibles, and trade names in the case of indefinite-lived intangibles. Finite-lived intangibles are amortized over their estimated useful lives and are tested for impairment whenever events or changes in circumstances indicate that the carrying value of the assets may not be recoverable. Indefinite lived intangible assets are tested for impairment annually, or as deemed necessary if potential indicators of impairment exist. The Company determined no impairment exists for the years ended December 31, 2022 and December 31, 2021. Warrant Valuation The Company generally accounts for warrants issued in connection with debt and equity financings as a component of equity, unless the warrants include a conditional obligation to issue a variable number of shares or there is a deemed possibility that it may need to settle the warrants in cash. Where there is a possibility that the Company may have to settle warrants in cash, it estimates the fair value of the issued warrants as a liability at each reporting date and record changes in the estimated fair value as a non-cash gain or loss in the consolidated statements of operations and comprehensive loss. The fair values of these warrants have been determined using the Black-Scholes option-pricing model (the “Black-Scholes Model”) and the Binomial Lattice model (the “Lattice Model”). The Black-Scholes Model requires inputs, such as the expected volatility, expected term, exercise price, risk-free interest rate, and the value of the underlying security. The Lattice Model provides for assumptions regarding expected volatility, expected term, exercise price, risk-free interest rates, the value of the underlying security, and the probability of and likely timing of a specific event within the period to maturity. These values are subject to a significant degree of the Company’s judgment. The Company’s common stock price represents a significant input that affects the valuation of the warrants. Going Concern The Company assesses its ability to continue as a going concern in accordance with ASC 205-40, Presentation of Financial Statements – Going Concern. The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. Revenue Recognition The Company records its revenue in accordance with ASC 606, Revenue from Contracts with Customers. Revenue is recognized upon transfer of control of promised products or services to customers in an amount that reflects the consideration the Company expects to receive in exchange for those products or services. The Company enters into contracts that can include various combinations of products and services, which when capable of being distinct, are accounted for as separate performance obligations. Revenue recognition is evaluated based on the following five steps: (i) identification of the contract with the customer; (ii) identification of the performance obligations in the contract; (iii) determination of the transaction price; (iv) allocation of the transaction price to the performance obligations in the contract; and (v) recognition of revenue when or as a performance obligation is satisfied. For multiple-element arrangements, revenue is allocated to each performance obligation based on its relative standalone selling price. Standalone selling prices are determined based on observable prices at which the Company separately sells its products or services. If a standalone selling price is not directly observable, judgment is made to estimate the selling price based on market conditions and entity-specific factors including cost plus analyses, features and functionality of the product and/or services, the geography of the Company’s customers, and type of customer. Any discounts or other reductions to the transaction price are allocated proportionately to all performance obligations within the multiple-element arrangement. The Company periodically validates the stand-alone selling price for performance obligations by evaluating whether changes in the key assumptions used to determine the stand-alone selling prices will have a significant effect on the allocation of transaction price between multiple performance obligations. The Company exercised judgement to determine that a product returns reserve was not required as historical returns activity have not been material. Table of Contents Ekso Bionics Holdings, Inc. Notes to Consolidated Financial Statements (In thousands, except per share amounts) 60 Research and Development Research and development costs consist of costs incurred for internal research and development activities. These costs primarily include salaries and other personnel-related expenses, contractor fees, prototype materials, facility costs, supplies, and depreciation of equipment associated with the design and development of new products prior to the establishment of their technological feasibility. Such costs are expensed as incurred. Income Taxes The Company accounts for income taxes using the asset and liability method. Under this method, income tax expense or benefit is recognized for the amount of taxes payable or refundable for the current year and for deferred tax liabilities and assets for the future tax consequences of events that have been recognized in the Company's consolidated financial statements or tax returns. The Company accounts for any income tax contingencies in accordance with accounting guidance for income taxes. The measurement of current and deferred tax assets and liabilities is based on provisions of currently enacted tax laws. The effects of any future changes in tax laws or rates have not been considered. For the preparation of the Company's consolidated financial statements included herein, the Company estimates its income taxes and tax contingencies in each of the tax jurisdictions in which it operates prior to the completion and filing of its tax returns. This process involves estimating actual current tax expense together with assessing temporary differences resulting from differing treatment of items, such as deferred revenue, for tax and accounting purposes. These differences result in net deferred tax assets and liabilities. The Company must then assess the likelihood that the deferred tax assets will be realizable, and to the extent they believe that realizability is not likely, the Company must establish a valuation allowance. In assessing the need for any additional valuation allowance, the Company considers all the evidence available to it, both positive and negative, including historical levels of income, legislative developments, expectations and risks associated with estimates of future taxable income, and ongoing prudent and feasible tax planning strategies. Stock-based Compensation The Company measures stock-based compensation expense for stock options granted to employees and directors based on the estimated fair value of the award on the date of grant and recognizes the fair value on a straight-line basis over the requisite service periods of the awards. The Company determines the fair value of stock options on the date of grant using the Black- Scholes Model, which is affected by the Company’s stock price and assumptions regarding a number of subjective variables. These variables include, but are not limited to, the Company’s stock price, volatility over the term of the awards, and actual and projected employee stock option exercise behaviors (expected term). Due to the lack of sufficient historical exercise data to provide a reasonable basis upon which to estimate expected term, the Company adopted the simplified method of estimating the expected term pursuant to SEC Staff Accounting Bulletin Topic 14. On this basis, the Company estimated the expected term of options granted by taking the average of the vesting term and the contractual term of the option. The Company measures stock-based compensation expense for restricted stock units (“RSUs”) and performance stock units ("PSUs") made to employees and directors based on the Company’s closing stock price on the date of grant and recognizes the value on a straight-line basis over the requisite service periods of the awards. The Company records compensation expense for service-based awards on a straight-line basis over the requisite service period, which is generally the vesting period of the award. For awards with performance-based conditions, at the point that it becomes probable that the performance conditions will be met, the Company records a cumulative catch-up of the expense from the grant date to the current date, and then amortizes the remainder of the expense over the remaining service period. Management evaluates when the achievement of a performance-based condition is probable based on the expected satisfaction of the performance conditions as of the reporting date. The amount of stock-based compensation expense recognized during a period is based on the value of the portion of the awards that are ultimately expected to vest. The Company accounts for forfeitures as they occur. The Company has, from time to time, modified the terms of its stock options to employees. The Company accounts for the incremental increase in the fair value over the original award on the date of the modification as an expense for vested awards or over the remaining service (vesting) period for unvested awards. The incremental compensation cost is the excess of the fair value of the modified award on the date of modification over the fair value of the original award immediately before the modification. Table of Contents Ekso Bionics Holdings, Inc. Notes to Consolidated Financial Statements (In thousands, except per share amounts) 61 Recent Accounting Pronouncements In June 2016, the FASB issued Accounting Standard Update ("ASU") No. 2016-13, Financial Instruments-Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments and subsequent amendments to the initial guidance under ASU 2018-19, ASU 2019-04, ASU 2019-05 and ASU 2019-10, which amends the current approach to estimate credit losses on certain financial assets, including trade and other receivables. Generally, this amendment requires entities to establish a valuation allowance for the expected lifetime losses of these certain financial assets. Upon the initial recognition of such assets, which will be based on, among other things, historical information, current conditions, and reasonable supportable forecasts. Subsequent changes in the valuation allowance are recorded in current earnings and reversal of previous losses are permitted. Currently, U.S. GAAP requires entities to write down credit losses only when losses are probable and loss reversals are not permitted. The Company will adopt ASU 2016-13 as of January 1, 2023, using the modified retrospective transition method. The adoption of ASU 2016-13 is not expected to have a material impact on the Company's financial position or the results of operations. In August 2020, the FASB issued ASU No. 2020-06, Accounting for Convertible Instruments and Contracts in an Entity's Own Equity, which simplifies the accounting for convertible instruments. ASU 2020-06 eliminates certain models that require separate accounting for embedded conversion features, in certain cases. Additionally, among other changes, the guidance eliminates certain of the conditions for equity classification for contracts in an entity’s own equity. The guidance also requires entities to use the if-converted method for all convertible instruments in the diluted earnings per share calculation and include the effect of share settlement for instruments that may be settled in cash or shares, except for certain liability-classified share- based payment awards. This guidance is effective for the Company beginning in the first quarter of 2024 and must be applied using either a modified or full retrospective approach. Early adoption is permitted. The Company does not expect the impact of adopting ASU 2020-06 to be material on its consolidated financial statements. 3. Net Loss Per Share of Common Stock Basic net loss per share of common stock is computed using the weighted average number of shares of common stock outstanding during the period. Diluted net loss per share is computed, when applicable, using the weighted average number of shares of common stock, adjusted to include conversion of "in-the-money" stock options and warrants for common stock and release of common stock in connection with restricted stock units during the period, net of tax as follows: Years ended December 31, 2022 2021 Numerator: Net loss $ (15,080) $ (9,764) Adjustment for gain on fair value of warrant liability — (1,029) Adjusted net loss used for dilution calculation $ (15,080) $ (10,793) Denominator Weighted-average number of shares outstanding 12,962 12,193 Effect of potential dilutive shares — 76 Dilutive weighted-average number of shares outstanding 12,962 12,269 Net loss per share Basic $ (1.16) $ (0.80) Diluted $ (1.16) $ (0.88) Table of Contents Ekso Bionics Holdings, Inc. Notes to Consolidated Financial Statements (In thousands, except per share amounts) 62 The following table sets forth potential shares of common stock that are not included in the calculation of diluted net loss per share because to do so would be anti-dilutive as of the end of each period presented: Years ended December 31, 2022 2021 Options to purchase common stock 270 491 Restricted stock units 1,383 655 Warrants for common stock 1,240 920 Total common stock equivalents 2,893 2,066 4. Human Motion and Control Acquisition On December 5, 2022, the Company acquired the human motion and control (“HMC”) business from Parker Hannifin Corporation (“Parker”), an Ohio corporation (the "HMC Acquisition"). The assets acquired from the business unit include intellectual property rights for devices which are FDA-cleared lower-limb powered exoskeletons that enable task-specific, overground gait training to patients with weakness or paralysis in their lower extremities. Products include Ekso Indego Personal, a light-weight exoskeleton for safe use in most home and community environments, and Ekso Indego Therapy, an adjustable exoskeleton for patients with spinal cord injury and stroke complementing Ekso’s product offering in outpatient facilities. The assets purchased by the Company include intellectual property related to the aforementioned Ekso Indego devices and future products in the orthotics and prosthetics space, inventories related to the Ekso Indego product line, fixed assets configured for the manufacture of the Ekso Indego products, and Ekso Indego devices maintained for service and sales demonstrations. The Company did not acquire any cash in connection with the acquisition of the business unit. As consideration for the assets acquired, the Company (i) paid the Seller $5,000 in cash and (ii) delivered to the Seller a $5,000 unsecured, subordinated zero percent interest promissory note (the “Promissory Note”). Under the terms of the Promissory Note, the Company shall pay the Seller sixteen (16) equal quarterly installments of $313, with the first payment being due and payable December 31, 2023, and the last payment being due and payable September 30, 2027. For additional information see Note 10. Notes Payable, Net in the notes to our consolidated financial statements included elsewhere in the Annual Report on Form 10-K. The Company accounted for the acquisition as a business combination in accordance with ASC 805, Business Combinations, by applying the acquisition method, and accordingly, the purchase price of $9,055, as calculated in the table below, was allocated to the assets acquired and liabilities assumed based on their fair values at the acquisition date. The fair values presented for fixed assets, intangible assets, and goodwill are preliminary figures pending final fair value analyses. In accordance with ASC 805, the acquirer has a year from the date of acquisition to recognize measurement period adjustments. The preliminary fair values presented below could be subject to change as result of the aforementioned adjustments. The excess of the purchase price over the preliminary net assets acquired of $431 was recorded as goodwill. The goodwill recognized is attributed primarily to expected synergies of HMC with the Company. From the acquisition date and as of December 31, 2022, there were no changes in the recognized amounts of goodwill resulting from the acquisition. The following table summarizes the preliminary fair values of the assets acquired, liabilities assumed and consideration given as of the acquisition date. These estimates are preliminary, pending final evaluation of certain assets, and therefore, are subject to revisions that may result in adjustments to the values presented below: Table of Contents Ekso Bionics Holdings, Inc. Notes to Consolidated Financial Statements (In thousands, except per share amounts) 63
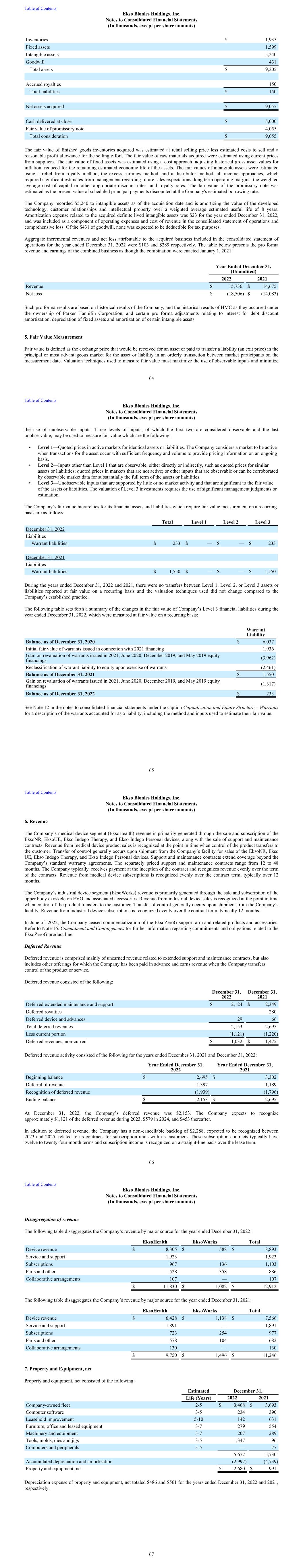
Inventories $ 1,935 Fixed assets 1,599 Intangible assets 5,240 Goodwill 431 Total assets $ 9,205 Accrued royalties 150 Total liabilities $ 150 Net assets acquired $ 9,055 Cash delivered at close $ 5,000 Fair value of promissory note 4,055 Total consideration $ 9,055 The fair value of finished goods inventories acquired was estimated at retail selling price less estimated costs to sell and a reasonable profit allowance for the selling effort. The fair value of raw materials acquired were estimated using current prices from suppliers. The fair value of fixed assets was estimated using a cost approach, adjusting historical gross asset values for inflation, reduced for the remaining estimated economic life of the assets. The fair values of intangible assets were estimated using a relief from royalty method, the excess earnings method, and a distributor method, all income approaches, which required significant estimates from management regarding future sales expectations, long term operating margins, the weighted average cost of capital or other appropriate discount rates, and royalty rates. The fair value of the promissory note was estimated as the present value of scheduled principal payments discounted at the Company's estimated borrowing rate. The Company recorded $5,240 to intangible assets as of the acquisition date and is amortizing the value of the developed technology, customer relationships and intellectual property over a weighted average estimated useful life of 8 years. Amortization expense related to the acquired definite lived intangible assets was $23 for the year ended December 31, 2022, and was included as a component of operating expenses and cost of revenue in the consolidated statement of operations and comprehensive loss. Of the $431 of goodwill, none was expected to be deductible for tax purposes. Aggregate incremental revenues and net loss attributable to the acquired business included in the consolidated statement of operations for the year ended December 31, 2022 were $103 and $289 respectively. The table below presents the pro forma revenue and earnings of the combined business as though the combination were enacted January 1, 2021: Year Ended December 31, (Unaudited) 2022 2021 Revenue $ 15,736 $ 14,675 Net loss $ (18,506) $ (14,083) Such pro forma results are based on historical results of the Company, and the historical results of HMC as they occurred under the ownership of Parker Hannifin Corporation, and certain pro forma adjustments relating to interest for debt discount amortization, depreciation of fixed assets and amortization of certain intangible assets. 5. Fair Value Measurement Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize Table of Contents Ekso Bionics Holdings, Inc. Notes to Consolidated Financial Statements (In thousands, except per share amounts) 64 the use of unobservable inputs. Three levels of inputs, of which the first two are considered observable and the last unobservable, may be used to measure fair value which are the following: • Level 1—Quoted prices in active markets for identical assets or liabilities. The Company considers a market to be active when transactions for the asset occur with sufficient frequency and volume to provide pricing information on an ongoing basis. • Level 2—Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. • Level 3—Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. The valuation of Level 3 investments requires the use of significant management judgments or estimation. The Company’s fair value hierarchies for its financial assets and liabilities which require fair value measurement on a recurring basis are as follows: Total Level 1 Level 2 Level 3 December 31, 2022 Liabilities Warrant liabilities $ 233 $ — $ — $ 233 December 31, 2021 Liabilities Warrant liabilities $ 1,550 $ — $ — $ 1,550 During the years ended December 31, 2022 and 2021, there were no transfers between Level 1, Level 2, or Level 3 assets or liabilities reported at fair value on a recurring basis and the valuation techniques used did not change compared to the Company’s established practice. The following table sets forth a summary of the changes in the fair value of Company’s Level 3 financial liabilities during the year ended December 31, 2022, which were measured at fair value on a recurring basis: Warrant Liability Balance as of December 31, 2020 $ 6,037 Initial fair value of warrants issued in connection with 2021 financing 1,936 Gain on revaluation of warrants issued in 2021, June 2020, December 2019, and May 2019 equity financings (3,962) Reclassification of warrant liability to equity upon exercise of warrants (2,461) Balance as of December 31, 2021 $ 1,550 Gain on revaluation of warrants issued in 2021, June 2020, December 2019, and May 2019 equity financings (1,317) Balance as of December 31, 2022 $ 233 See Note 12 in the notes to consolidated financial statements under the caption Capitalization and Equity Structure – Warrants for a description of the warrants accounted for as a liability, including the method and inputs used to estimate their fair value. Table of Contents Ekso Bionics Holdings, Inc. Notes to Consolidated Financial Statements (In thousands, except per share amounts) 65 6. Revenue The Company’s medical device segment (EksoHealth) revenue is primarily generated through the sale and subscription of the EksoNR, EksoUE, Ekso Indego Therapy, and Ekso Indego Personal devices, along with the sale of support and maintenance contracts. Revenue from medical device product sales is recognized at the point in time when control of the product transfers to the customer. Transfer of control generally occurs upon shipment from the Company’s facility for sales of the EksoNR, Ekso UE, Ekso Indego Therapy, and Ekso Indego Personal devices. Support and maintenance contracts extend coverage beyond the Company’s standard warranty agreements. The separately priced support and maintenance contracts range from 12 to 48 months. The Company typically receives payment at the inception of the contract and recognizes revenue evenly over the term of the contracts. Revenue from medical device subscriptions is recognized evenly over the contract term, typically over 12 months. The Company’s industrial device segment (EksoWorks) revenue is primarily generated through the sale and subscription of the upper body exoskeleton EVO and associated accessories. Revenue from industrial device sales is recognized at the point in time when control of the product transfers to the customer. Transfer of control generally occurs upon shipment from the Company’s facility. Revenue from industrial device subscriptions is recognized evenly over the contract term, typically 12 months. In June of 2022, the Company ceased commercialization of the EksoZeroG support arm and related products and accessories. Refer to Note 16. Commitment and Contingencies for further information regarding commitments and obligations related to the EksoZeroG product line. Deferred Revenue Deferred revenue is comprised mainly of unearned revenue related to extended support and maintenance contracts, but also includes other offerings for which the Company has been paid in advance and earns revenue when the Company transfers control of the product or service. Deferred revenue consisted of the following: December 31, 2022 December 31, 2021 Deferred extended maintenance and support $ 2,124 $ 2,349 Deferred royalties — 280 Deferred device and advances 29 66 Total deferred revenues 2,153 2,695 Less current portion (1,121) (1,220) Deferred revenues, non-current $ 1,032 $ 1,475 Deferred revenue activity consisted of the following for the years ended December 31, 2021 and December 31, 2022: Year Ended December 31, 2022 Year Ended December 31, 2021 Beginning balance $ 2,695 $ 3,302 Deferral of revenue 1,397 1,189 Recognition of deferred revenue (1,939) (1,796) Ending balance $ 2,153 $ 2,695 At December 31, 2022, the Company’s deferred revenue was $2,153. The Company expects to recognize approximately $1,121 of the deferred revenue during 2023, $579 in 2024, and $453 thereafter. In addition to deferred revenue, the Company has a non-cancellable backlog of $2,288, expected to be recognized between 2023 and 2025, related to its contracts for subscription units with its customers. These subscription contracts typically have twelve to twenty-four month terms and subscription income is recognized on a straight-line basis over the lease term. Table of Contents Ekso Bionics Holdings, Inc. Notes to Consolidated Financial Statements (In thousands, except per share amounts) 66 Disaggregation of revenue The following table disaggregates the Company’s revenue by major source for the year ended December 31, 2022: EksoHealth EksoWorks Total Device revenue $ 8,305 $ 588 $ 8,893 Service and support 1,923 — 1,923 Subscriptions 967 136 1,103 Parts and other 528 358 886 Collaborative arrangements 107 — 107 $ 11,830 $ 1,082 $ 12,912 The following table disaggregates the Company’s revenue by major source for the year ended December 31, 2021: EksoHealth EksoWorks Total Device revenue $ 6,428 $ 1,138 $ 7,566 Service and support 1,891 — 1,891 Subscriptions 723 254 977 Parts and other 578 104 682 Collaborative arrangements 130 — 130 $ 9,750 $ 1,496 $ 11,246 7. Property and Equipment, net Property and equipment, net consisted of the following: Estimated December 31, Life (Years) 2022 2021 Company-owned fleet 2-5 $ 3,468 $ 3,693 Computer software 3-5 234 390 Leasehold improvement 5-10 142 631 Furniture, office and leased equipment 3-7 279 554 Machinery and equipment 3-7 207 289 Tools, molds, dies and jigs 3-5 1,347 96 Computers and peripherals 3-5 — 77 5,677 5,730 Accumulated depreciation and amortization (2,997) (4,739) Property and equipment, net $ 2,680 $ 991 Depreciation expense of property and equipment, net totaled $486 and $561 for the years ended December 31, 2022 and 2021, respectively. Table of Contents Ekso Bionics Holdings, Inc. Notes to Consolidated Financial Statements (In thousands, except per share amounts) 67
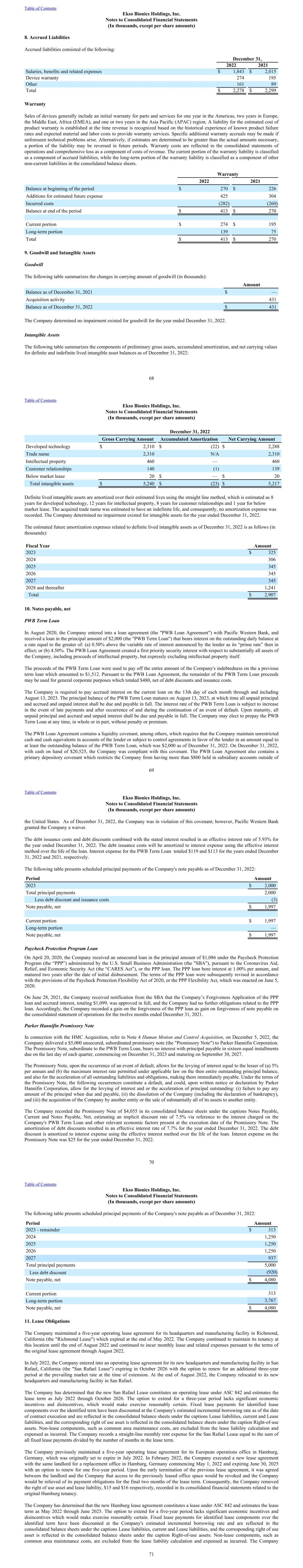
8. Accrued Liabilities Accrued liabilities consisted of the following: December 31, 2022 2021 Salaries, benefits and related expenses $ 1,843 $ 2,015 Device warranty 274 195 Other 161 89 Total $ 2,278 $ 2,299 Warranty Sales of devices generally include an initial warranty for parts and services for one year in the Americas, two years in Europe, the Middle East, Africa (EMEA), and one or two years in the Asia Pacific (APAC) region. A liability for the estimated cost of product warranty is established at the time revenue is recognized based on the historical experience of known product failure rates and expected material and labor costs to provide warranty services. Specific additional warranty accruals may be made if unforeseen technical problems arise. Alternatively, if estimates are determined to be greater than the actual amounts necessary, a portion of the liability may be reversed in future periods. Warranty costs are reflected in the consolidated statements of operations and comprehensive loss as a component of costs of revenue. The current portion of the warranty liability is classified as a component of accrued liabilities, while the long-term portion of the warranty liability is classified as a component of other non-current liabilities in the consolidated balance sheets. Warranty 2022 2021 Balance at beginning of the period $ 270 $ 226 Additions for estimated future expense 425 304 Incurred costs (282) (260) Balance at end of the period $ 413 $ 270 Current portion $ 274 $ 195 Long-term portion 139 75 Total $ 413 $ 270 9. Goodwill and Intangible Assets Goodwill The following table summarizes the changes in carrying amount of goodwill (in thousands): Amount Balance as of December 31, 2021 $ — Acquisition activity 431 Balance as of December 31, 2022 $ 431 The Company determined no impairment existed for goodwill for the year ended December 31, 2022. Intangible Assets The following table summarizes the components of preliminary gross assets, accumulated amortization, and net carrying values for definite and indefinite lived intangible asset balances as of December 31, 2022: Table of Contents Ekso Bionics Holdings, Inc. Notes to Consolidated Financial Statements (In thousands, except per share amounts) 68 December 31, 2022 Gross Carrying Amount Accumulated Amortization Net Carrying Amount Developed technology $ 2,310 $ (22) $ 2,288 Trade name 2,310 N/A 2,310 Intellectual property 460 — 460 Customer relationships 140 (1) 139 Below market lease 20 $ — $ 20 Total intangible assets $ 5,240 $ (23) $ 5,217 Definite lived intangible assets are amortized over their estimated lives using the straight line method, which is estimated as 8 years for developed technology, 12 years for intellectual property, 8 years for customer relationships and 1 year for below market lease. The acquired trade name was estimated to have an indefinite life, and consequently, no amortization expense was recorded. The Company determined no impairment existed for intangible assets for the year ended December 31, 2022. The estimated future amortization expenses related to definite lived intangible assets as of December 31, 2022 is as follows (in thousands): Fiscal Year Amount 2023 $ 325 2024 306 2025 345 2026 345 2027 345 2028 and thereafter 1,241 Total $ 2,907 10. Notes payable, net PWB Term Loan In August 2020, the Company entered into a loan agreement (the "PWB Loan Agreement") with Pacific Western Bank, and received a loan in the principal amount of $2,000 (the "PWB Term Loan") that bears interest on the outstanding daily balance at a rate equal to the greater of: (a) 0.50% above the variable rate of interest announced by the lender as its “prime rate” then in effect; or (b) 4.50%. The PWB Loan Agreement created a first priority security interest with respect to substantially all assets of the Company, including proceeds of intellectual property, but expressly excluding intellectual property itself. The proceeds of the PWB Term Loan were used to pay off the entire amount of the Company's indebtedness on the a previous term loan which amounted to $1,512. Pursuant to the PWB Loan Agreement, the remainder of the PWB Term Loan proceeds may be used for general corporate purposes which totaled $480, net of debt discounts and issuance costs. The Company is required to pay accrued interest on the current loan on the 13th day of each month through and including August 13, 2023. The principal balance of the PWB Term Loan matures on August 13, 2023, at which time all unpaid principal and accrued and unpaid interest shall be due and payable in full. The interest rate of the PWB Term Loan is subject to increase in the event of late payments and after occurrence of and during the continuation of an event of default. Upon maturity, all unpaid principal and accrued and unpaid interest shall be due and payable in full. The Company may elect to prepay the PWB Term Loan at any time, in whole or in part, without penalty or premium. The PWB Loan Agreement contains a liquidity covenant, among others, which requires that the Company maintain unrestricted cash and cash equivalents in accounts of the lender or subject to control agreements in favor of the lender in an amount equal to at least the outstanding balance of the PWB Term Loan, which was $2,000 as of December 31, 2022. On December 31, 2022, with cash on hand of $20,525, the Company was compliant with this covenant. The PWB Loan Agreement also contains a primary depository covenant which restricts the Company from having more than $800 held in subsidiary accounts outside of Table of Contents Ekso Bionics Holdings, Inc. Notes to Consolidated Financial Statements (In thousands, except per share amounts) 69 the United States. As of December 31, 2022, the Company was in violation of this covenant; however, Pacific Western Bank granted the Company a waiver. The debt issuance costs and debt discounts combined with the stated interest resulted in an effective interest rate of 5.93% for the year ended December 31, 2022. The debt issuance costs will be amortized to interest expense using the effective interest method over the life of the loan. Interest expense for the PWB Term Loan totaled $119 and $113 for the years ended December 31, 2022 and 2021, respectively. The following table presents scheduled principal payments of the Company's note payable as of December 31, 2022: Period Amount 2023 $ 2,000 Total principal payments 2,000 Less debt discount and issuance costs (3) Note payable, net $ 1,997 Current portion $ 1,997 Long-term portion — Note payable, net $ 1,997 Paycheck Protection Program Loan On April 20, 2020, the Company received an unsecured loan in the principal amount of $1,086 under the Paycheck Protection Program (the “PPP”) administered by the U.S. Small Business Administration (the "SBA"), pursuant to the Coronavirus Aid, Relief, and Economic Security Act (the “CARES Act”), or the PPP loan. The PPP loan bore interest at 1.00% per annum, and matured two years after the date of initial disbursement. The terms of the PPP loan were subsequently revised in accordance with the provisions of the Paycheck Protection Flexibility Act of 2020, or the PPP Flexibility Act, which was enacted on June 5, 2020. On June 28, 2021, the Company received notification from the SBA that the Company’s Forgiveness Application of the PPP loan and accrued interest, totaling $1,099, was approved in full, and the Company had no further obligations related to the PPP loan. Accordingly, the Company recorded a gain on the forgiveness of the PPP loan as gain on forgiveness of note payable on the consolidated statement of operations for the twelve months ended December 31, 2021. Parker Hannifin Promissory Note In connection with the HMC Acquisition, refer to Note 4 Human Motion and Control Acquisition, on December 5, 2022, the Company delivered a $5,000 unsecured, subordinated promissory note (the "Promissory Note") to Parker Hannifin Corporation. The Promissory Note, subordinate to the PWB Term Loan, bears no interest with principal payable in sixteen equal installments due on the last day of each quarter, commencing on December 31, 2023 and maturing on September 30, 2027. The Promissory Note, upon the occurrence of an event of default, allows for the levying of interest equal to the lesser of (a) 5% per annum and (b) the maximum interest rate permitted under applicable law on the then entire outstanding principal balance, and also for the acceleration of all outstanding liabilities and obligations, making them immediately payable. Under the terms of the Promissory Note, the following occurrences constitute a default, and could, upon written notice or declaration by Parker Hannifin Corporation, allow for the levying of interest and or the acceleration of principal outstanding: (i) failure to pay any amount of the principal when due and payable, (ii) the dissolution of the Company (including the declaration of bankruptcy), and (iii) the acquisition of the Company by another entity or the sale of substantially all of its assets to another entity. The Company recorded the Promissory Note of $4,055 in its consolidated balance sheets under the captions Notes Payable, Current and Notes Payable, Net, estimating an implicit discount rate of 7.5% via reference to the interest charged on the Company's PWB Term Loan and other relevant economic factors present at the execution date of the Promissory Note. The amortization of debt discounts resulted in an effective interest rate of 7.7% for the year ended December 31, 2022. The debt discount is amortized to interest expense using the effective interest method over the life of the loan. Interest expense on the Promissory Note was $25 for the year ended December 31, 2022. Table of Contents Ekso Bionics Holdings, Inc. Notes to Consolidated Financial Statements (In thousands, except per share amounts) 70 The following table presents scheduled principal payments of the Company's note payable as of December 31, 2022: Period Amount 2023 - remainder $ 313 2024 1,250 2025 1,250 2026 1,250 2027 937 Total principal payments 5,000 Less debt discount (920) Note payable, net $ 4,080 Current portion 313 Long-term portion 3,767 Note payable, net $ 4,080 11. Lease Obligations The Company maintained a five-year operating lease agreement for its headquarters and manufacturing facility in Richmond, California (the "Richmond Lease") which expired at the end of May 2022. The Company continued to maintain its tenancy at this location until the end of August 2022 and continued to incur monthly lease and related expenses pursuant to the terms of the original lease agreement through August 2022. In July 2022, the Company entered into an operating lease agreement for its new headquarters and manufacturing facility in San Rafael, California (the "San Rafael Lease") expiring in October 2026 with the option to renew for an additional three-year period at the prevailing market rate at the time of extension. At the end of August 2022, the Company relocated to its new headquarters and manufacturing facility in San Rafael. The Company has determined that the new San Rafael Lease constitutes an operating lease under ASC 842 and estimates the lease term as July 2022 through October 2026. The option to extend for a three-year period lacks significant economic incentives and disincentives, which would make exercise reasonably certain. Fixed lease payments for identified lease components over the identified term have been discounted at the Company's estimated incremental borrowing rate as of the date of contract execution and are reflected in the consolidated balance sheets under the captions Lease liabilities, current and Lease liabilities, and the corresponding right of use asset is reflected in the consolidated balance sheets under the caption Right-of-use assets. Non-lease components, such as common area maintenance costs, are excluded from the lease liability calculation and expensed as incurred. The Company records a straight-line monthly rent expense for the San Rafael Lease equal to the sum of all fixed lease payments divided by the number of months in the lease term. The Company previously maintained a five-year operating lease agreement for its European operations office in Hamburg, Germany, which was originally set to expire in July 2022. In February 2022, the Company executed a new lease agreement with the same landlord for a replacement office in Hamburg, Germany commencing May 1, 2022 and expiring June 30, 2025 with an option to renew for one five-year period. Upon the early termination of the previous lease agreement, it was agreed between the landlord and the Company that access to the previously leased office space would be revoked and the Company would be relieved of its payment obligations for the final two months of the lease term. Consequently, the Company removed the right of use asset and lease liability, $15 and $16 respectively, recorded in its consolidated financial statements related to the original Hamburg tenancy. The Company has determined that the new Hamburg lease agreement constitutes a lease under ASC 842 and estimates the lease term as May 2022 through June 2025. The option to extend for a five-year period lacks significant economic incentives and disincentives which would make exercise reasonably certain. Fixed lease payments for identified lease components over the identified term have been discounted at the Company's estimated incremental borrowing rate and are reflected in the consolidated balance sheets under the captions Lease liabilities, current and Lease liabilities, and the corresponding right of use asset is reflected in the consolidated balance sheets under the caption Right-of-use assets. Non-lease components, such as common area maintenance costs, are excluded from the lease liability calculation and expensed as incurred. The Company Table of Contents Ekso Bionics Holdings, Inc. Notes to Consolidated Financial Statements (In thousands, except per share amounts) 71
records a straight-line monthly rent expense for this lease equal to the sum of all fixed lease payments divided by the number of months in the lease term. The Company’s future lease payments as of December 31, 2022 are as follows, which are presented as Lease liabilities, current and Lease liabilities on the Company’s consolidated balance sheets: Period Operating Leases 2023 $ 408 2024 420 2025 401 2026 349 Total lease payments 1,578 Less: imputed interest (150) Present value of lease liabilities $ 1,428 Lease liabilities, current $ 341 Lease liabilities 1,087 Total lease liabilities $ 1,428 Weighted-average remaining term (in years) 3.7 Weighted-average discount rate 5.4 % Lease expense under the Company’s operating leases was $605 and $527, for the years ended December 31, 2022 and 2021, respectively. 12. Employee Benefit Plan The Company administers a 401(k) retirement plan, or the 401(k) Plan, in which all employees are eligible to participate. Each eligible employee may elect to contribute to the 401(k) Plan. The Company makes matching contributions in the form of shares of the Company's common stock to the 401(k) Plan in an amount equal to 50% of employee contributions (up to the statutory limit), subsequent to year-end. The expense related to the contribution was $186 and $171 for the years ended December 31, 2022 and 2021, respectively. 13. Capitalization and Equity Structure Summary The Company’s authorized capital stock at December 31, 2022 consisted of 141,429 shares of common stock and 10,000 shares of preferred stock. As of December 31, 2022, there were 13,203 shares of common stock outstanding and no shares of preferred stock issued and outstanding. Common Stock The holders of outstanding shares of common stock are entitled to receive dividends out of assets or funds legally available for the payment of dividends at such times and in such amounts as the Board of Directors may determine. Holders of common stock are entitled to one vote for each share held on all matters submitted to a vote of stockholders. There is no cumulative voting for the election of directors. The common stock is not entitled to preemptive rights and is not subject to conversion or redemption. Upon liquidation, dissolution or winding up of the Company, the assets legally available for distribution to stockholders are distributable ratably among the holders of the common stock after payment of liquidation preferences, if any, on any outstanding payment of other claims of creditors. Each outstanding share of common stock is duly and validly issued, fully paid, and non-assessable. Table of Contents Ekso Bionics Holdings, Inc. Notes to Consolidated Financial Statements (In thousands, except per share amounts) 72 February 2021 Offering In February 2021, the Company entered into an amended and restated underwriting agreement (the "Underwriting Agreement") with H.C. Wainwright & Co., LLC ("Wainwright"), to sell 3,902 shares of the Company's common stock for a public price of $10.25 per share, for gross proceeds of $40,000 (the "February 2021 Offering"). The Company received net proceeds of $36,504 from the February 2021 Offering after deducting underwriting discounts, commissions and offering expenses. Pursuant to the Underwriting Agreement, the Company issued, to certain designees of Wainwright, five year warrants (the “2021 Warrants”) to purchase shares of the Company's common stock in an amount equal to 7.0% of the aggregate number of shares sold in the February 2021 Offering, or 273 shares, at an exercise price of $12.81 per share. At the Market Offering In October 2020, the Company entered into an At The Market Offering Agreement (the "ATM Agreement") with H.C. Wainwright & Co., LLC (the "Agent"), under which the Company may issue and sell shares of its common stock, from time to time, to or through the Agent. Offers and sales of shares of common stock by the Company through the Agent may be made by any method deemed to be an “at the market offering” as defined under SEC Rule 415 or in privately negotiated transactions, subject to certain conditions. Such shares may be offered pursuant to the registration statement on Form S-3 (File No. 333-239203) (the “Registration Statement”), which was declared effective by the SEC on June 26, 2020, and a related prospectus supplement filed with the SEC on October 9, 2020 (the “ATM Prospectus”). Pursuant to the Registration Statement and the ATM Prospectus, shares having an aggregate offering price of up to $7,500 may be offered and sold, subject to certain SEC rules limiting the amount of shares of the Company’s common stock that may be sold by the Company under the Registration Statement. Under the ATM Agreement, shares of the Company's common stock may not be sold for a price lower than $6.75 per share. During the year ended December 31, 2022, the Company did not sell any shares under the ATM Agreement. As of December 31, 2022, the Company has $6,668 available for future offerings under the prospectus filed with respect to the ATM Agreement. Preferred Stock The Company may issue shares of preferred stock from time to time in one or more series, each of which will have such distinctive designation or title as shall be determined by its Board of Directors and will have such voting powers, full or limited, or no voting powers, and such preferences and relative, participating, optional or other special rights and such qualifications, limitations or restrictions thereof, as shall be stated in such resolution or resolutions providing for the issue of such class or series of preferred stock as may be adopted from time to time by the Board of Directors. Warrants Warrant shares outstanding as of December 31, 2022 and December 31, 2021 were as follows: Source Exercise Price Term (Years) December 31, 2021 Issued Expired Exercised December 31, 2022 2021 Warrants $ 12.81 5 273 — — — 273 June 2020 Investor Warrants $ 5.18 5.5 127 — — — 127 June 2020 Placement Agent Warrants $ 5.64 5 39 — — — 39 December 2019 Warrants $ 8.10 5 556 — — — 556 December 2019 Placement Agent Warrants $ 8.44 5 52 — — — 52 May 2019 Warrants $ 3.52 3 193 — — — 193 1,240 — — — 1,240 During the years ended December 31, 2022 and 2021, the Company received net proceeds of $0 and $1,417 from the exercise of 0 and 358 warrants and issued 0 and 300 shares of common stock, respectively, as a result of those exercises. The weighted average exercise price of the warrants outstanding as of December 31, 2022 was $8.06. 2021 Warrants Table of Contents Ekso Bionics Holdings, Inc. Notes to Consolidated Financial Statements (In thousands, except per share amounts) 73 In February 2021, the Company issued the 2021 Warrants, exercisable for up to 273 shares of the Company’s common stock at an exercise price of $12.81 per share. The 2021 Warrants were issued as exercisable immediately, and will expire five years from the date of issuance, or on February 11, 2026. In addition, the 2021 Warrants contain a cashless exercise provision, whereby, if, at the time a holder exercises its 2021 Warrants, a registration statement registering the issuance or the resale of the shares of common stock underlying the 2021 Warrants under the Securities Act is not then effective or available for the issuance of such shares, then in lieu of making the cash payment otherwise contemplated to be made to the Company upon such exercise in payment of the aggregate exercise price, the holder may elect to instead receive, upon such exercise (either in whole or in part), the net number of shares of the Company’s common stock determined according to a formula set forth in the 2021 Warrants. The 2021 Warrants will be automatically exercised on a cashless basis on their expiration date. The 2021 Warrants could also require payment of liquidated damages by the Company in the form of cash payments in the event of a failure by the Company to timely deliver shares of common stock upon exercise of such warrants. For the years ended December 31, 2021 and December 31, 2022, no shares of the 2021 Warrants were exercised. The 2021 Warrants also contain a put option, under which, if the Company enters into a Fundamental Transaction, as defined in the 2021 Warrants, the Company or any successor entity will, at the option of a holder of a 2021 Warrant, exercisable concurrently with or at any time within 30 days after the consummation of such Fundamental Transaction, purchase such holder’s 2021 Warrant by paying to such holder an amount of cash equal to the Black-Scholes value of the remaining unexercised portion of such holder’s 2021 Warrant within five trading days after the notice of exercise by the holder of the put option. Because of this put option provision, the 2021 Warrants are classified as a liability and are marked to market at each reporting date. The warrant liability related to the 2021 Warrants is measured at fair value upon issuance and at each reporting date using certain estimated inputs, which are classified within Level 3 of the fair value hierarchy. The following assumptions were used in the Black-Scholes Model to measure the fair value of the 2021 Warrants: December 31, 2022 December 31, 2021 Current share price $ 1.19 $ 2.65 Conversion price $ 12.81 $ 12.81 Risk-free interest rate 4.21 % 1.13 % Expected term (years) 3.11 4.11 Volatility of stock 99.6 % 98.3 % June 2020 Investor Warrants In June 2020, the Company issued the June 2020 Investor Warrants, exercisable for up to 874 shares of the Company’s common stock at an exercise price of $5.18 per share. The June 2020 Warrants were issued as exercisable immediately, and will expire five and one-half years from the date of issuance, or on December 10, 2025. In addition, the June 2020 Investor Warrants contain a cashless exercise provision, whereby, if, at the time a holder exercises its June 2020 Investor Warrants, a registration statement registering the issuance or the resale of the shares of common stock underlying the June 2020 Investor Warrants under the Securities Act is not then effective or available for the issuance of such shares, then in lieu of making the cash payment otherwise contemplated to be made to the Company upon such exercise in payment of the aggregate exercise price, the holder may elect to instead receive, upon such exercise (either in whole or in part), the net number of shares of the Company’s common stock determined according to a formula set forth in the June 2020 Investor Warrant. The June 2020 Investor Warrants will be automatically exercised on a cashless basis on their expiration date. The June 2020 Investor Warrants could also require payment of liquidated damages by the Company in the form of cash payments in the event of a failure by the Company to timely deliver shares of common stock upon exercise of such warrants. For the years ended December 31, 2021 and December 31, 2022, 270 and no shares of the June 2020 Investor Warrants were exercised respectively. Table of Contents Ekso Bionics Holdings, Inc. Notes to Consolidated Financial Statements (In thousands, except per share amounts) 74 The June 2020 Investor Warrants also contain a put option, under which, if the Company enters into a Fundamental Transaction, as defined in the June 2020 Investor Warrants, the holders of the June 2020 Investor Warrants will be entitled to receive upon exercise of the June 2020 Investor Warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the June 2020 Investor Warrants immediately prior to such Fundamental Transaction. Alternatively, the Company or any successor entity will, at the option of a holder of a June 2020 Investor Warrant, exercisable concurrently with or at any time within 30 days after the consummation of such Fundamental Transaction, purchase such holder’s June 2020 Investor Warrant by paying to such holder an amount of cash equal to the Black-Scholes value of the remaining unexercised portion of such holder’s June 2020 Investor Warrant. Because of this put-option provision, the June 2020 Investor Warrants are classified as a liability and are marked to market at each reporting date. The warrant liability related to the June 2020 Investor Warrants is measured at fair value upon issuance and at each reporting date using certain estimated inputs, which are classified within Level 3 of the fair value hierarchy. The following assumptions were used in the Black-Scholes Model to measure the fair value of the June 2020 Investor Warrants: December 31, 2022 December 31, 2021 Current share price $ 1.19 $ 2.65 Conversion price $ 5.18 $ 5.18 Risk-free interest rate 4.23 % 1.11 % Expected term (years) 2.94 3.94 Volatility of stock 99.6 % 103.9 % June 2020 Placement Agent Warrants In June 2020, the Company issued the June 2020 Placement Agent Warrants, exercisable for up to 122 shares of the Company’s common stock, to the placement agent for such offering. The June 2020 Placement Agent Warrants have substantially the same form as the June 2020 Investor Warrants, including the put option described above, except that they have an exercise price per share equal to $5.64, subject to adjustment in certain circumstances, and will expire on June 7, 2025. For the years ended December 31, 2021 and December 31, 2022, 83 and no shares of the June 2020 Investor Warrants were exercised respectively. Because of the put-option provision in the June 2020 Placement Agent Warrants, these warrants are classified as a liability and are marked to market at each reporting date. The warrant liability related to the June 2020 Placement Agent Warrants is measured at fair value at each reporting date using certain estimated inputs, which are classified within Level 3 of the fair value hierarchy. The following assumptions were used in the Black-Scholes Model to measure the fair value of the June 2020 Placement Agent Warrants: December 31, 2022 December 31, 2021 Current share price $ 1.19 $ 2.65 Conversion price $ 5.64 $ 5.64 Risk-free interest rate 4.33 % 1.03 % Expected term (years) 2.44 3.44 Volatility of stock 73.5 % 100.0 % December 2019 Warrants In December 2019, pursuant to a securities purchase agreement (the "December 2019 Offering") the Company issued warrants (the "December 2019 Warrants") to purchase 556 shares of common stock. The December 2019 Warrants are currently exercisable and have an exercise price of $8.10 per share, and will expire five years from the date they initially became exercisable, or on June 21, 2025. The December 2019 Warrants also contain a put option, under which, if the Company enters into a Fundamental Transaction, as defined in the December 2019 Warrants, the Company or any successor entity will, at the option of a holder of a December Table of Contents Ekso Bionics Holdings, Inc. Notes to Consolidated Financial Statements (In thousands, except per share amounts) 75
2019 Warrant, exercisable concurrently with or at any time within 30 days after the consummation of such Fundamental Transaction, purchase such holder’s December 2019 Warrant by paying to such holder an amount of cash equal to the Black- Scholes value of the remaining unexercised portion of such holder’s December 2019 Warrant within five trading days after the notice of exercise by the holder of the put option. Because of this put-option provision, the December 2019 Warrants are classified as a liability and are marked to market at each reporting date. For the years ended December 31, 2021 and December 31, 2022, zero shares of the December 2019 Warrants were exercised. The warrant liability related to the December 2019 Warrants is measured at fair value at each reporting date using certain estimated inputs, which are classified within Level 3 of the fair value hierarchy. The following assumptions were used in the Black-Scholes Model to measure the fair value of the December 2019 Warrants: December 31, 2022 December 31, 2021 Current share price $ 1.19 $ 2.65 Conversion price $ 8.10 $ 8.10 Risk-free interest rate 4.32 % 1.04 % Expected term (years) 2.47 3.47 Volatility of stock 73.3 % 99.7 % December 2019 Placement Agent Warrants In December 2019, in connection with the December 2019 Offering, the Company issued warrants to purchase 52 shares of the Company’s common stock to the placement agent for such offering (the "December 2019 Placement Agent Warrants"). The December 2019 Placement Agent Warrants have substantially the same form as the December 2019 Warrants, except that they have an exercise price per share equal to $8.44, subject to adjustment in certain circumstances, and will expire on December 18, 2025. For the years ended December 31, 2021 and December 31, 2022, zero shares of the December 2019 Placement Agent Warrants were exercised. The warrant liability related to the December 2019 Placement Agent Warrants is measured at fair value at each reporting date using certain estimated inputs, which are classified within Level 3 of the fair value hierarchy. The following assumptions were used in the Black-Scholes Model to measure the fair value of the December 2019 Placement Agent Warrants: December 31, 2022 December 31, 2021 Current share price $ 1.19 $ 2.65 Conversion price $ 8.44 $ 8.44 Risk-free interest rate 4.42 % 0.96 % Expected term (years) 1.97 2.97 Volatility of stock 71.8 % 102.9 % Management has assessed that the likelihood of a Change of Control (as defined in the December 2019 Placement Agent Warrants) occurring during the term of the December 2019 Placement Agent Warrants is low, and that if such an event were to occur, the difference between the cashless exercise value and the warrants fair value is nominal. May 2019 Warrants In May 2019, pursuant to an underwriting agreement, (the "May 2019 Offering"), the Company issued warrants (the "May 2019 Warrants") to purchase 444 shares of common stock. The May 2019 Warrants are currently exercisable and have a current exercise price of $3.52 per share, and will expire five years from the date of their issuance, or on May 24, 2024. The May 2019 Warrants contain a price protection feature, pursuant to which, subject to certain exceptions, if shares of common stock are sold or issued in the future, or securities convertible or exercisable for shares of the Company’s common stock are sold or issued in the future, for consideration, or with an exercise price or conversion price, as applicable, per share less than the exercise price Table of Contents Ekso Bionics Holdings, Inc. Notes to Consolidated Financial Statements (In thousands, except per share amounts) 76 per share then in effect for the May 2019 Warrants, the exercise price of the May 2019 Warrants is reduced to the consideration paid for, or the exercise price or conversion price of, as the case may be, the securities issued in such offering. Pursuant to this provision, in connection with the June 2020 Offering, the exercise price of the May 2019 Warrants was reduced to $3.52 per share, being the amount that is equal to the lower of (x) the consideration paid for the securities issued in the June 2020 Offering, or $4.51 per share, (y) the lowest exercise price of the June 2020 Warrants, or $5.18, and (z) the lowest one-day volume-weighted average price of the Company’s Common Stock on the Nasdaq Capital Market as measured each day during the five trading day period starting on June 8, 2020, rounded to the nearest share, or $3.52. In addition, if the Company effects or enters into any issuance of common stock or options or convertible securities exercisable for or convertible into common stock at a price which varies or may vary with the market price of the shares of the Company's common stock, subject to certain exceptions, a May 2019 Warrant holder may, at the time of exercise of the holder’s warrant, elect to exercise the warrant at such variable price. The May 2019 Warrants include a put option, whereby while the May 2019 Warrants are outstanding, if the Company enters into a Change of Control, as defined in the May 2019 Warrants, the Company or any successor entity will, at the option of a 2019 Warrant holder exercise within 90 days after the public disclosure of the Change of Control transaction, purchase such holder’s May 2019 Warrants by paying to such holder an amount of cash equal to the Black-Scholes value of the remaining unexercised portion of such warrants on the later date of consummation of the Change of Control transaction or two trading days after the notice of such request. Because of this put option provision, the May 2019 Warrants are classified as a liability and are marked to market at each reporting date. For the years ended December 31, 2021 and December 31, 2022, 5 and no shares of the May 2019 Warrants were exercised respectively. The warrant liability related to the May 2019 Warrants is measured at fair value at each reporting and exercise date using certain estimated inputs, which are classified within Level 3 of the fair value hierarchy. Because of the price protection feature contained in the May 2019 Warrants, the Company uses a combination of the Black-Scholes Model and the Lattice Model to estimate the fair value of the warrants at each reporting period. The following assumptions were used in the Black-Scholes Model in combination with the Lattice Model to measure the fair value of the May 2019 Warrants: December 31, 2022 December 31, 2021 Current share price $ 1.19 $ 2.65 Conversion price $ 3.52 $ 3.52 Risk-free interest rate 4.6 % 0.83 % Expected term (years) 1.40 2.40 Volatility of stock 74.5 % 109.1 % Management has assessed that the likelihood of a Change of Control occurring during the term of the warrants is low, and that if such an event were to occur, the difference between the cashless exercise value and the May 2019 Warrants fair value is nominal. 14. Stock-based Compensation 2014 Equity Incentive Plan In 2014, the Board of Directors and a majority of the stockholders adopted the 2014 Equity Incentive Plan, or the 2014 Plan, allowing for the issuance of 137 shares of common stock. The 2014 Plan has since been amended and restated with approval by the stockholders to increase the maximum number of shares issuable, as shown in the table below: Table of Contents Ekso Bionics Holdings, Inc. Notes to Consolidated Financial Statements (In thousands, except per share amounts) 77 Original share pool 137 2015 increase 111 June 2017 increase 67 December 2017 increase (ratified in June 2018) 293 2019 increase 233 March 2020 increase 333 December 2020 increase 800 June 2022 increase 550 Total share authorized for grant as of December 31, 2022 2,524 As of December 31, 2022, the total shares authorized for grant under the 2014 Plan was 2,524, of which 50 were available for future grants. Under the terms of the 2014 Plan, the Board of Directors may award stock options, restricted stock, restricted stock units, stock appreciation rights and dividend equivalent rights having either a fixed or variable price related to the fair market value of the shares and with an exercise or conversion privilege related to the passage of time, the occurrence of one or more events, or the satisfaction of performance criteria or other conditions or any other security with the value derived from the value of the shares. Shares available for future grant under the 2014 Plan was as follows: Shares Available For Grant Available as of December 31, 2021 587 Share pool increase 550 Granted (1,499) Forfeited 243 Expired 169 Available as of December 31, 2022 50 Stock Options The Board of Directors may grant stock options under the 2014 Plan at a price of not less than 100% of the fair market value of the Company’s common stock on the date the option is granted. The maximum term of an incentive stock option granted to participants may not exceed ten years. Subject to the limitations discussed above, the Board of Directors determines the term and exercise or purchase price of other awards granted under the 2014 Plan. The Board of Directors also determines the terms and conditions of awards, including the vesting schedule and any forfeiture provisions. Options granted under the 2014 Plan vest upon the passage of time, generally four years, or upon the attainment of certain performance criteria established by the Board of Directors. The Company may grant options to purchase common stock to non-employees for advisory and consulting services. Upon exercise of a stock option, the Company issues new shares of common stock. A summary of the stock option activity during the year ended December 31, 2022 is presented below: Options Outstanding Weighted Average Exercise Price Weighted Average Remaining Contractual Life (Years) Aggregate Intrinsic Value Outstanding at beginning of year 491 $ 32.53 Forfeited (51) $ 12.66 Expired (170) $ 29.86 Outstanding at end of year 270 $ 37.96 5.26 $ — Vested and expected to vest 270 $ 37.96 5.26 $ — Exercisable at year end 261 $ 38.93 5.21 $ — Table of Contents Ekso Bionics Holdings, Inc. Notes to Consolidated Financial Statements (In thousands, except per share amounts) 78 No stock options were exercised during the years ended December 31, 2022 and 2021. As no stock options were granted during the years ended December 31, 2022 and December 31, 2021, there was no related weighted-average grant date fair value. The total grant date fair value of stock options vested during the years ended December 31, 2022 and 2021 was $428 and $1,194, respectively. As of December 31, 2022, total unrecognized compensation cost related to unvested stock options was $57. This amount is expected to be recognized as stock-based compensation expense in the Company’s consolidated statements of operations and comprehensive loss over the remaining weighted average vesting period of 0.83 years. The following table summarizes information about stock options outstanding as of December 31, 2022: Options Outstanding Options Exercisable Range of Exercise Prices Number of Shares Weighted- Average Remaining Contractual Life (Years) Weighted Average Price Number of Shares Weighted Average Price $5.55 - $9.15 98 7 $ 6.66 90 $ 6.51 $16.95 - $27.90 60 5.36 $ 23.52 59 $ 23.52 $30.75 - $85.50 74 4.87 $ 39.75 75 $ 39.75 $101.85 - $229.95 38 1.85 $ 138.41 37 $ 138.41 270 5.26 $ 37.96 261 $ 38.93 The Company recognizes compensation expense using the straight-line method over the requisite service period. Restricted Stock Units The Company issues time-based RSUs and PSUs to employees and non-employee service providers. Each RSU and PSU represents the right to receive one share of the Company’s common stock upon vesting and subsequent settlement. PSUs vest upon achievement of performance targets based on the Company's annual operating plan. The fair values of RSUs and PSUs are determined based on the closing price of the Company’s common stock on the date of grant. Combined RSU and PSU activity for the year ended December 31, 2022 is summarized below: Number of Shares Weighted Average Grant- Date Fair Value Unvested as of January 1, 2022 655 $ 5.63 Granted 1,499 $ 1.76 Vested (579) $ 3.93 Forfeited (192) $ 5.52 Unvested as of December 31, 2022 1,383 $ 2.17 The total grant-date fair value of RSUs and PSUs that vested during the year ended December 31, 2022 was $1,081. As of December 31, 2022, $2,215 of total unrecognized compensation expense related to unvested RSUs and PSUs was expected to be recognized over a weighted average period of 1.41 years. Compensation Expense Stock-based compensation is included in the consolidated statements of operations and comprehensive loss in general and administrative, research and development, or sales and marketing expenses, depending upon the nature of services provided. Stock-based compensation expense related to stock options, RSUs and PSUs was recorded as follows: Table of Contents Ekso Bionics Holdings, Inc. Notes to Consolidated Financial Statements (In thousands, except per share amounts) 79

Years Ended December 31, 2022 2021 Sales and marketing $ 263 $ 450 Research and development 339 270 General and administrative 1,944 1,609 $ 2,546 $ 2,329 Employee Stock Purchase Plan The Company has an Employee Stock Purchase Plan, or ESPP. Under the ESPP, the Company has 500 shares of common stock reserved for issuance, subject to adjustment in the event of a stock split, stock dividend, combination or reclassification or similar event. The ESPP allows eligible employees to purchase shares of the Company’s common stock at a discount through payroll deductions of up to 25% of their eligible compensation, subject to any plan limitations. The ESPP provides for six- month offering periods. At the end of each offering period, employees can purchase shares at 85% of the lower of the fair market value of the Company’s common stock on the first trading day of the offering period or on the last trading day of the offering period. As of December 31, 2022, the Company had not initiated employee enrollment to the plan. 15. Income Taxes The domestic and foreign components of pre-tax loss for the years ended December 31, 2022 and 2021 were as follows: Years Ended December 31, 2022 2021 Domestic $ (13,749) $ (9,069) Foreign (1,331) (695) Loss before income taxes $ (15,080) $ (9,764) The Company had no current or deferred federal and state income tax expense or benefit for the years ended December 31, 2022 and 2021 because the Company generated net operating losses, and currently management does not believe it is more likely than not that the net operating losses will be realized. The Company’s non-U.S. tax obligation is primarily for business activities conducted through Germany and Singapore for which taxes were included in other expense, net for the years ended December 31, 2022 and 2021 and determined to be immaterial and accordingly, such amounts were excluded from the following tables. Income tax expense (benefit) for the years ended December 31, 2022 and 2021 differed from the amounts computed by applying the statutory federal income tax rate of 21% to pretax loss as a result of the following: Years Ended December 31, 2022 2021 Federal tax at statutory rate 21.0 % 21.0 % State tax, net of federal tax effect — — R&D credit 0.7 0.4 Change in valuation allowance (15.1) (31.3) Unrealized gain on warrant 1.8 8.5 PPP Loan Forgiveness — 2.4 Stock-based compensation (7.7) (2.8) Other (1.8) 0.9 Foreign exchange 1.1 0.9 Total tax expense (benefit) — % — % Table of Contents Ekso Bionics Holdings, Inc. Notes to Consolidated Financial Statements (In thousands, except per share amounts) 80 The tax effects of temporary differences and related deferred tax assets and liabilities as of December 31, 2022 and 2021 were as follows: December 31, 2022 2021 Deferred tax assets: Depreciation and other $ 249 $ 257 Net operating loss carryforwards 48,829 47,579 Research and development tax credits 2,034 1,899 Accruals and reserves 356 395 Capitalized research and development costs 640 — Deferred revenue 213 377 Stock compensation expense 1,670 2,763 Lease assets 236 30 Other 22 20 Deferred tax liabilities: Lease liabilities (208) (28) Prepaid expenses (41) (32) Less: Valuation allowance (54,000) (53,260) Net deferred tax asset (liability) $ — $ — The Company’s accounting for deferred taxes involves the evaluation of a number of factors concerning the realizability of the Company’s net deferred tax assets. The Company primarily considered such factors as the Company’s history of operating losses, the nature of the Company’s deferred tax assets, and the timing, likelihood and amount, if any, of future taxable income during the periods in which those temporary differences and carryforwards become deductible. The Company does not believe that it is more likely than not that the deferred tax assets will be realized; accordingly, a full valuation allowance was established and no deferred tax assets were shown in the accompanying consolidated balance sheets. The valuation allowance increased by $740 and $4,587 in the years ended December 31, 2022 and December 31, 2021, respectively. For tax years beginning after December 31, 2018, the Global Intangible Low-taxed Income ("GILTI") took effect. Due to the aggregated losses of the foreign subsidiaries, there was no GILTI inclusion for the years ended December 31, 2022 and December 31, 2021. The Tax Cuts and Jobs Act of 2017 (TCJA) made a significant change to Section 174 that went into effect for taxable years beginning after December 31, 2021. The change eliminated the ability to currently deduct research and development costs. Instead, these costs must be capitalized and amortized. As a result, the Company capitalized research and development costs of $3.3 million for the years ended December 31, 2022. On March 27, 2020 the U.S. enacted the Coronavirus Aid, Relief, and Economic Security Act (the CARES Act). On December 21, 2020, The U.S. Congress passed the Consolidation Appropriations Act, 2021 (the CAA Act). The Company evaluated the provisions of the CARES Act and CCA Act and determined that it did not result in a significant impact on its tax provision. On June 29, 2020 California Assembly Bill 85 (AB 85) was signed into law, which suspended the use of California net operating losses and limits the use of California research tax credits for tax years beginning in 2020 and before 2023. However, on February 9, 2022 California Senate Bill 113 (SB 113) was signed into law and removed the limitation on the net operating losses and credits for the 2022 year and allows, after taxable years beginning on or after January 1, 2022, the ability to utilize net operating losses and credits. These changes did not result in a significant impact on the value of the Company's deferred tax assets. As of December 31, 2022 the Company had federal net operating loss carryforwards of $186,722. The federal net operating loss carryforwards of $120,792 generated before January 1, 2018 will begin to expire in 2027, and $65,930 will carryforward Table of Contents Ekso Bionics Holdings, Inc. Notes to Consolidated Financial Statements (In thousands, except per share amounts) 81 indefinitely but are subject to the 80% taxable income limitation. The Company also had federal research and development tax credit carryforwards of $2,142 that will expire beginning in 2031, if not utilized. As of December 31, 2022, the Company had state net operating loss carryforwards of $120,724, which will begin to expire in 2028. The Company also had state research and development tax credit carryforwards of $723, which have no expiration. As of December 31, 2022, the Company had foreign net operating loss carryforwards of $11,650. The foreign net operating loss carryforwards do not expire. Utilization of the Company’s net operating losses and credit carryforwards may be subject to annual limitations in the event of a Section 382 ownership change. Such future limitations could result in the expiration of net operating losses and credit carryforwards before utilization as a result of such an ownership change. A reconciliation of the beginning and ending amount of unrecognized tax benefits for the years ended December 31, 2022 and 2021, were as follows: Years Ended December 31, 2022 2021 Beginning balances as of January 1, 2022 and 2021 $ 668 $ 645 Increase of unrecognized tax benefits taken in prior years — 1 Increase of unrecognized tax benefits related to current year 48 22 Ending balances as of December 31, 2022 and 2021 $ 716 $ 668 If the Company is able to recognize these uncertain tax positions, the unrecognized tax benefits would not impact the effective tax rate if the Company applies a full valuation allowance against the deferred tax assets, as provided in the Company’s current policy. The Company had not incurred any material tax interest or penalties as of December 31, 2022. The Company does not anticipate any significant change within 12 months of this reporting date of its uncertain tax positions. The Company is subject to taxation in the United States and various state jurisdictions, Germany, and Singapore. There are no ongoing examinations by taxing authorities at this time. The Company’s tax years 2007 through 2022 will remain open for examination by the federal and state authorities for three and four years, respectively, from the date of utilization of any net operating loss credits. The Company’s 2017 to 2022 tax years will remain open for examination by the German tax authority for four years from the end of the year in which the applicable return was filed. The Company’s 2018 to 2022 tax years will remain open for examination by the Singapore tax authority for four years from the date of the applicable assessment. 16. Commitments and Contingencies Commitments Material Contracts The Company has two license agreements with the Regents of the University of California to maintain exclusive rights to patents. The Company is required to pay 1% of net sales of licensed medical devices sold to entities other than the U.S. government. In addition, the Company is required to pay 21% of consideration collected from any sub-licensee for the grant of the sub-license. The Company entered into a research and development collaboration agreement in December 2021 with a party that develops technologies having utility in robotic exoskeletons from research and development activities associated with a specific set of government funded research projects. Since January 2022, the Company has assisted with research and development activities in exchange for access to a worldwide, royalty free, transferable, sublicensable, exclusive license to design and market products that use or incorporate the jointly-developed technology within Ekso’s target market segments. In connection with the HMC Acquisition, the Company assumed two license agreements with Vanderbilt University to maintain exclusive rights to patents on the Company's behalf. The Vanderbilt Exoskeleton License Agreement was entered into Table of Contents Ekso Bionics Holdings, Inc. Notes to Consolidated Financial Statements (In thousands, except per share amounts) 82 as of October 15, 2012 and will continue until April 29, 2038, unless sooner terminated. Under this agreement, the Company is required to pay 6% of net sales of licensed patent products and 3% of net sales of licensed software products and the minimum annual royalty for licensed products is $250,000 after July 31, 2018. The Vanderbilt Knee License Agreement was entered into as of March 1, 2022 and will continue until February 15, 2041, unless sooner terminated. Under this agreement, the Company is required to pay 3.75% of net sales for licensed patent products and the minimum annual royalty is $75,000 due on or before July 31, 2028 and $100,000 after that. In addition to the assumption of the license agreements, the Company entered into transitional use agreements with Parker granting the Company access to certain information technology systems and manufacturing facilities in Macedonia, Ohio for twelve months following the date of the acquisition. As consideration for access to these resources, the Company will make monthly payments of $20. Purchase Obligations The Company purchases components from a variety of suppliers and uses contract manufacturers to provide manufacturing services for its products. Purchase obligations are defined as agreements that are enforceable and legally binding and that specify all significant terms, including: fixed or minimum quantities to be purchased; fixed, minimum or variable price provisions; and the approximate timing of the transaction. Due to a variety of factors, including the COVID-19 pandemic, various materials the Company used to manufacture its products are currently experiencing shortages and supply chain disruptions. Electronic components in general, semiconductor chips, battery cells, metals and plastics, all of which are used in the Company's products, are also in shorter supply compared to prior periods, and the Company is also experiencing longer lead times for manufacturing services such as machining and tool making and increased pricing. Numerous factors, such as the ongoing pandemic or further trade tensions between the United States and China, may prolong or deepen these challenges. The Company had purchase obligations primarily for purchases of inventory and manufacturing related service contracts totaling $3,480 as of December 31, 2022, which are expected to be paid within one year. Timing of payments and actual amounts paid may be different depending on the time of receipt of goods or services or changes to agreed-upon amounts for some obligations. Timing of payments and actual amounts paid may be different depending on the time of receipt of goods or services or changes to agreed-upon amounts for some obligations. The Company has operating lease commitments totaling $1,578 payable over 45 months related to the San Rafael and Hamburg leases disclosed in Note 11. Lease Obligations. Other Contractual Obligations The following table summarizes the Company's outstanding contractual obligations, including interest payments, as of December 31, 2022 and the effect those obligations are expected to have on its liquidity and cash flows in future periods: Payments Due By Period Total Less than one year 1-3 Years 3-5 Years Term loan $ 2,107 $ 2,107 $ — $ — Promissory Note 5,000 313 2,500 2,187 Facility operating leases 1,578 408 821 349 Total $ 8,685 $ 2,828 $ 3,321 $ 2,536 Contingencies In the normal course of business, the Company is subject to various legal matters. In the opinion of management, the resolution of such matters will not have a material adverse effect on the Company’s consolidated financial statements. 17. Segment Disclosures The Company has two reportable segments: EksoHealth and EksoWorks. The EksoHealth segment designs, engineers, manufactures, and markets exoskeletons for applications in the medical markets. The EksoWorks segment designs, engineers, manufactures, and markets exoskeleton devices to allow able-bodied users to perform difficult repetitive work for extended periods. The reportable segments are each managed separately because they serve distinct markets. Table of Contents Ekso Bionics Holdings, Inc. Notes to Consolidated Financial Statements (In thousands, except per share amounts) 83

The Company evaluates performance and allocates resources based on segment gross profit margin. The Company does not consider net assets as a segment measure and, accordingly, assets are not allocated. Segment reporting information is as follows: EksoHealth EksoWorks Total Year ended December 31, 2022 Revenue $ 11,830 $ 1,082 $ 12,912 Cost of revenue 5,949 749 6,698 Gross profit $ 5,881 $ 333 $ 6,214 Year ended December 31, 2021 Revenue $ 9,750 $ 1,496 $ 11,246 Cost of revenue 3,746 751 4,497 Gross profit $ 6,004 $ 745 $ 6,749 Geographically, the regions the Company operates in are the Americas, EMEA, and APAC. Individual countries with revenue greater than 10% of total revenue are disclosed separately from the regional totals. Geographic information for revenue based on location of customers is as follows: Year ended December 31, 2022 2021 United States $ 6,557 $ 6,451 Other 252 127 Americas 6,809 6,578 Germany 1,002 1,327 Other 2,847 2,084 EMEA 3,849 3,411 APAC 2,254 1,257 $ 12,912 $ 11,246 18. Related Party Transactions On February 4, 2023, the Company entered into a mutual release and settlement agreement with an entity to settle and resolve any and all potential claims brought forth in connection with a consulting agreement executed between itself and the Company in July of 2017. Under the terms of the consulting agreement, the Company was required to make milestone payments for the introduction of potential partners for, and the consummation of, a strategic joint venture. A member of the Company's board of directors is affiliated with one of two entities under common control. In connection with the settlement agreement, the Company recorded $205 in general and administrative operating expenses for the year ended December 31, 2022 and has recorded a liability of $325 in its consolidated balance sheet as of December 31, 2022. There were no expenses or liabilities recorded related to the settlement agreement for the year ended December 31, 2021. The total settlement amount of $325 is expected to be paid in cash over the next fourteen months. Table of Contents Ekso Bionics Holdings, Inc. Notes to Consolidated Financial Statements (In thousands, except per share amounts) 84 Item 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE None. Item 9A. CONTROLS AND PROCEDURES Disclosure Controls and Procedures. Our management, with the participation of our principal executive officer and principal financial officer, conducted an evaluation of our disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) as of December 31, 2022. Based upon that evaluation, our principal executive officer and principal financial officer concluded that, as of such date, our disclosure controls and procedures were effective to ensure that information required to be disclosed in reports filed by us under the Securities Exchange Act is recorded, processed, summarized and reported within the required time periods and is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate to allow timely decisions regarding required disclosure. It should be noted that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment and makes assumptions about the likelihood of future events. There can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions, regardless of how remote. Management believes that the financial statements included in this Annual Report fairly present in all material respects our financial condition, results of operations and cash flows for the periods presented. Management’s Report on Internal Control Over Financial Reporting Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in the U.S. Securities Exchange Act, Rules 13a-15(f) and 15d-15(f). Our internal control system was designed to provide reasonable assurance to our management and board of directors regarding the preparation and fair presentation of published financial statements. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2022 based on the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control— Integrated Framework (2013). Our management believes that based on this criteria, as of December 31, 2022, our internal control over financial reporting is effective. This Annual Report does not include an attestation report of our registered public accounting firm regarding our internal control over financial reporting. Our report was not subject to attestation by our registered public accounting firm pursuant to rules of the Securities and Exchange Commission that permits us to provide only management’s report in this Annual Report on Form 10-K. Changes in Internal Control Over Financial Reporting: There were no changes in our internal control over financial reporting identified in connection with the evaluation required by (d) of Exchange Act Rules 13a-15 or 15d-15 that occurred during our most recent fiscal quarter that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting. ITEM 9B. OTHER INFORMATION None. ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS Table of Contents 85 PART III Item 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE The information required by this Item is incorporated herein by reference from our Proxy Statement, relating to our 2023 Annual Meeting of Shareholders, under the heading “Corporate Governance,” to be filed with the SEC within 120 days of December 31, 2022. Item 11. EXECUTIVE COMPENSATION The information required by this Item is incorporated herein by reference from our Proxy Statement, relating to our 2023 Annual Meeting of Shareholders, under the headings “Executive Compensation” and “Director Compensation,” to be filed with the SEC within 120 days of December 31, 2022. Item 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS The information required by this Item is incorporated herein by reference from our Proxy Statement, relating to our 2023 Annual Meeting of Shareholders, under the heading “Ownership of our Common Stock,” to be filed with the SEC within 120 days of December 31, 2022. Item 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE The information required by this Item is incorporated herein by reference from our Proxy Statement, relating to our 2023 Annual Meeting of Shareholders, under the heading “Certain Relationships and Related Party Transactions,” to be filed with the SEC within 120 days of December 31, 2022. Item 14. PRINCIPAL ACCOUNTING FEES AND SERVICES The information required by this Item is incorporated herein by reference from our Proxy Statement, relating to our 2023 Annual Meeting of Shareholders, under the headings “Audit Committee Report” and “Audit Fees and Services,” to be filed with the SEC within 120 days of December 31, 2022. Table of Contents 86 PART IV Item 15. EXHIBITS, FINANCIAL STATEMENTS AND FINANCIAL STATEMENT SCHEDULES (a) Financial Statements and Schedules: The following financial statement documents are included as part of Item 8 to this Form 10-K: Report of Independent Registered Public Accounting Firm Consolidated Balance Sheets as of December 31, 2022 and 2021 Consolidated Statements of Operations and Comprehensive loss for the years ended December 31, 2022 and 2021 Consolidated Statements of Stockholders’ Equity for the years ended December 31, 2022 and 2021 Consolidated Statements of Cash Flows for the years ended December 31, 2022 and 2021 Notes to the Consolidated Financial Statements All schedules are omitted because they are not applicable or the required information is shown in the financial statements or notes thereto. (b) Exhibits. The exhibits filed with this Annual Report are set forth in the Exhibit Index. Table of Contents 87

Exhibit Index Exhibit Number Description 2.1# Asset Purchase Agreement between the Registrant and Parker Hannifin Corporation, dated as of December 5, 2022 (incorporated by reference from Exhibit 2.1 to the Registrant's Current Report on Form 8-K filed on December 5, 2022) 3.1 Articles of Incorporation of the Registrant (incorporated by reference from Exhibit 3.1 to the Registrant’s Annual Report on Form 10-K filed on March 19, 2015) 3.2 Certificate of Merger of Ekso Bionics, Inc., with and into Acquisition Sub, filed January 15, 2014 (incorporated by reference from Exhibit 3.3 to the Registrant’s Current Report on Form 8-K filed on January 23, 2014) 3.3 By-Laws of the Registrant (incorporated by reference from Exhibit 3.1 to the Registrant’s Current Report on Form 8-K filed on April 16, 2021) 3.4 Certificate of Designation of Preferences, Rights and Limitations of Series A Convertible Preferred Stock, filed on December 23, 2015 (incorporated by reference from Exhibit 3.1 to the Registrant’s Current Report on Form 8-K filed on December 24, 2015) 3.5 Certificate of Amendment to Certificate of Designation of Series A Convertible Preferred Stock, filed on April 4, 2016 (incorporated by reference from Exhibit 3.1 to the Registrant’s Current Report on Form 8-K filed on April 7, 2016) 3.6 Certificate of Change of Ekso Bionics Holdings, Inc. effective May 4, 2016 (incorporated by reference from Exhibit 3.1 to the Registrant’s Current Report on Form 8-K filed on May 5, 2016) 3.7 Certificate of Amendment of Certificate of Incorporation of Ekso Bionics Holdings, Inc. (incorporated by reference from Exhibit 3.1 to the Registrant’s Current Report on Form 8-K filed on December 27, 2017) 3.8 Certificate of Amendment of Certificate of Incorporation of Ekso Bionics Holdings, Inc. (incorporated by reference from Exhibit 3.1 to the Registrant’s Current Report on Form 8-K filed on March 24, 2020) 4.1 Form of specimen certificate (incorporated by reference from Exhibit 4.4 to the Registrant’s Registration Statement on Form S-3 filed on June 23, 2015) 4.2 Form of Amendment to Common Stock Purchase Warrant (incorporated by reference from Exhibit 99.2 to the Registrant’s Current Report on Form 8-K filed March 11, 2019) 4.3 Form of Common Stock Purchase Warrant (incorporated by reference from Exhibit 4.1 to the Registrant’s Current Report on Form 8-K filed December 20, 2019) 4.4 Form of Placement Agent Common Stock Purchase Warrant (incorporated by reference from Exhibit 4.2 to the Registrant’s Current Report on Form 8-K filed December 20, 2019) 4.5 Form of Warrant (incorporated by reference from Exhibit 4.1 to the Registrant’s Current Report on Form 8-K filed December 30, 2019) 4.6 Form of Warrant (incorporated by reference from Exhibit 4.1 to the Registrant’s Current Report on Form 8-K filed June 10, 2020) 4.7 Form of Placement Agent Warrant (incorporated by reference from Exhibit 4.2 to the Registrant’s Current Report on Form 8-K filed June 10, 2020) Table of Contents 88 4.8 Subordinated Promissory Note between Ekso Bionics Holdings, Inc. and Parker Hannifin Corporation, dated as of December 5, 2022 (incorporated by reference from Exhibit 4.1 to the Registrant’s Current Report on Form 8-K filed December 5, 2022) 4.9* Description of Registrant’s Securities Registered Pursuant to Section 12 of the Securities Exchange Act of 1934 4.10 Form of Underwriter Common Stock Purchase Warrant (incorporated by reference from Exhibit 4.1 to the Registrant’s Current Report on Form 8-K filed February 11, 2021) 10.1 At The Market Offering Agreement, by and among Ekso Bionics Holdings, Inc., and H.C. Wainwright & Co., LLC (incorporated by reference from Exhibit 1.1 to the Registrant’s Current Report on Form 8-K filed on October 9, 2020) 10.2 Form of Registration Rights Agreement (incorporated by reference from Exhibit 10.10 of the Registrant’s Current Report on Form 8-K filed on January 23, 2014) 10.3† Amended and Restated 2014 Equity Incentive Plan (incorporated by reference from Appendix A to the Registrant’s Proxy Statement on Schedule 14A filed on April 30, 2019) 10.4† Form of Director Option Agreement under 2014 Equity Incentive Plan (incorporated by reference from Exhibit 10.13 to the Registrant’s Current Report on Form 8-K filed on January 23, 2014) 10.5† Form of Employee Option Agreement under 2014 Equity Incentive Plan (incorporated by reference from Exhibit 10.14 to the Registrant’s Current Report on Form 8-K filed on January 23, 2014) 10.6† Form of Employee Restricted Stock Unit Award under 2014 Equity Incentive Plan (incorporated by reference from Exhibit 10.46 to the Registrant’s Quarterly Report on Form 10-Q filed August 7, 2017) 10.7† 2017 Employee Stock Purchase Plan (incorporated by reference from Appendix A to Registrant’s Proxy Statement on Schedule 14 filed on April 28, 2017) 10.8† Scott Davis Offer Letter dated February 22, 2021 (incorporated by reference from Exhibit 10.3 to the Registrant’s Current Report on Form 8-K filed January 21, 2022) 10.9†** Jason Jones Offer Letter dated September 19, 2018 (incorporated by reference from Exhibit 10.11 to the Registrant's Annual Report on Form 10-K filed February 27, 2020) 10.10† Executive Chair Employment Agreement by and between the Registrant and Steven Sherman (incorporated by reference from Exhibit 10.1 to the Registrant’s Current Report on Form 8-K filed December 5, 2022) 10.11†* Jerome Wong Officer Offer letter, dated October 26, 2022 10.12 Exclusive License Agreement, dated as of November 15, 2005, by and between The Regents of the University of California and Berkeley ExoTech, Inc., d/b/a Berkeley ExoWorks (incorporated by reference from Exhibit 10.19 to the Registrant’s Current Report on Form 8-K filed on January 23, 2014) 10.13 Exclusive License Agreement, dated as of July 14, 2008, by and between The Regents of the University of California and Berkeley ExoTech, Inc., d/b/a/ Berkeley Bionics and formerly d/b/a Berkeley ExoWorks (as amended by Amendment #1 to Exclusive License Agreement, dated as of May 20, 2009, by and between The Regents of the University of California and Berkeley Bionics) (incorporated by reference from Exhibit 10.20 to the Registrant’s Current Report on Form 8-K filed on January 23, 2014) Table of Contents 89 10.14* License Agreement between Vanderbilt University and Parker Hannifin Corporation, dated as of October 15, 2012 (as amended by the first amendment dated as of June 15, 2014, the second amendment dated as of December 1, 2018, and the third amendment dated as of May 1, 2019) 10.15* License Agreement between Vanderbilt University and Parker Hannifin Corporation dated as of March 1, 2022 10.16* Vanderbilt Assignment and Assumption Agreement between Ekso Bionics Holdings, Inc and Parker Hannifin Corporation, dated as of December 5, 2022 10.17† Form of Non-Employee Director Indemnification Agreement (incorporated by reference from Exhibit 10.20 to the Registrant’s Quarterly Report on Form 10-Q filed on May 13, 2014) 10.18† Form of Executive Officer Indemnification Agreement (incorporated by reference from Exhibit 10.21 to the Registrant’s Quarterly Report on Form 10-Q filed on May 13, 2014) 10.19 Form of Amendment to Purchase Agreement (incorporated by reference from Exhibit 99.1 to the Registrant’s Current Report on Form 8-K filed March 11, 2019) 10.2 Form of Securities Purchase Agreement (incorporated by reference from Exhibit 10.1 to the Registrant’s Current Report on Form 8-K filed December 20, 2019) 10.21 Loan and Security Agreement dated as of August 17, 2020 by and among the Registrant, EKSO Bionics Holdings, Inc., EKSO Bionics, Inc. and Pacific Western Bank (incorporated by reference from Exhibit 10.1 to the Registrant’s Current Report on Form 8-K filed August 21, 2020) 10.22* Lease, dated July 15, 2022, between Don Tornberg and Ekso Bionics Inc. 10.23* Transitional Use Agreement, dated December 5, 2022, between Parker Hannifin Corporation and Ekso Bionics Holdings, Inc. 21.1* Subsidiaries of the Registrant 23.1* Consent of Independent Registered Public Accounting Firm (WithumSmith+Brown, PC) 24.1 Power of attorney (included on signature page of this report) 31.1* Certification of Chief Executive Officer pursuant to Rule 13a-14(a) under the Securities Exchange Act of 1934, as amended. 31.2* Certification of Chief Financial Officer pursuant to Rule 13a-14(a) under the Securities Exchange Act of 1934, as amended. 32.1§ Certification of Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. 32.2§ Certification of Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. Table of Contents 90 101 Interactive Data Files of Financial Statements and Notes. 101.ins Instant Document 101.sch XBRL Taxonomy Schema Document 101.cal XBRL Taxonomy Calculation Linkbase Document 101.def XBRL Taxonomy Definition Linkbase Document 101.lab XBRL Taxonomy Label Linkbase Document 101.pre XBRL Taxonomy Presentation Linkbase Document 104 Cover Page Interactive Data File (formatted as inline XBRL and contained in Exhibit 101) # Certain of the exhibits and schedules to this exhibit have been omitted in accordance with Regulation S-K Item 601(a)(5). The Company agrees to furnish supplementally a copy of all omitted exhibits and schedules to the Securities and Exchange Commission upon its request. * Filed herewith ** Confidential Treatment portions of this exhibit have been omitted as permitted by applicable regulations. § The certifications attached as Exhibits 32.1 and 32.2 that accompany this Annual Report on Form 10-K are not deemed filed with the Securities and Exchange Commission and are not to be incorporated by reference into any filing of the Registrant under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date of this Annual Report on Form 10-K, irrespective of any general incorporation language contained in such filing. † Management contract or compensatory plan or arrangement Item 16. FORM 10-K SUMMARY The Company has elected not to include summary information. Table of Contents 91

SIGNATURES Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this Annual Report to be signed on its behalf by the undersigned, thereunto duly authorized. By: /S/ Scott G. Davis March 28, 2023 Scott G. Davis President and Chief Executive Officer POWERS OF ATTORNEY KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and Scott G. Davis and Jerome Wong, and each of them, as his true and lawful attorney-in-fact and agent, with full power of substitution and resubstitution, for him and in his name, place and stead, in any and all capacities, to sign any and all amendments to this Annual Report, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them or their substitutes may lawfully do or cause to be done by virtue hereof. Pursuant to the requirements of the Securities Exchange Act of 1934, this Annual Report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated. Signature Title Date /S/ Scott G. Davis President and Chief Executive Officer March 28, 2023 Scott G. Davis (Principal Executive Officer) /S/ Jerome Wong Chief Financial Officer March 28, 2023 Jerome Wong (Principal Accounting and Financial Officer) /S/ Mary Ann Cloyd Director March 28, 2023 Mary Ann Cloyd /S/ Corinna Lathan Director March 28, 2023 Corinna Lathan, Ph.D. /S/ Charles Li Director March 28, 2023 Charles Li, Ph.D. /S/ Steven Sherman Director March 28, 2023 Steven Sherman /S/ Stanley Stern Director March 28, 2023 Stanley Stern /S/ Rhonda A. Wallen Director March 28, 2023 Rhonda A. Wallen Table of Contents 92